The principle of preventing the deterioration and oxidation of ionomers in hydrogen production through anion-exchange membrane water electrolyzers (AEMWEs) has been discovered for the first time. This breakthrough is expected to enhance both the performance and durability of hydrogen production devices.
A research team, led by Professor Seung Geol Lee in the Department of Materials Science and Engineering at UNIST has introduced a novel AEMWE technology that employs an inexpensive non-platinum metal catalyst. By enabling potassium to adhere to the catalyst surface, this method minimizes direct contact with the ionomer, potentially reducing the cost of hydrogen production.
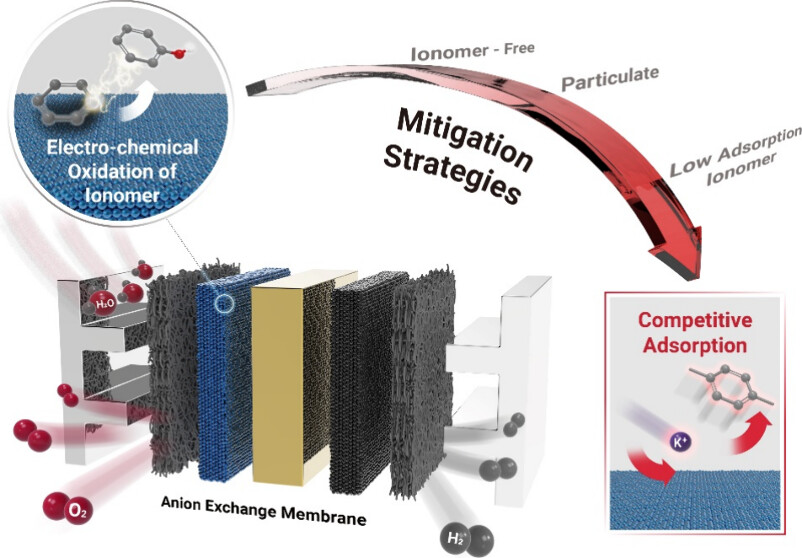 Figure 1. Schematic diagram of the overall strategy for the electrochemical oxidation prevention of ionomers.
Figure 1. Schematic diagram of the overall strategy for the electrochemical oxidation prevention of ionomers.
In typical hydrogen production devices, the properties of ions that facilitate ionic transport tend to degrade over time, resulting in decreased hydrogen production efficiency and a shortened device lifespan.
The research team capitalized on the fact that the adsorption energy of potassium is more than three times greater than that of organic compounds. Their findings indicate that substances such as potassium hydroxide and sodium hydroxide can significantly improve the performance and stability of the AEMWE system.
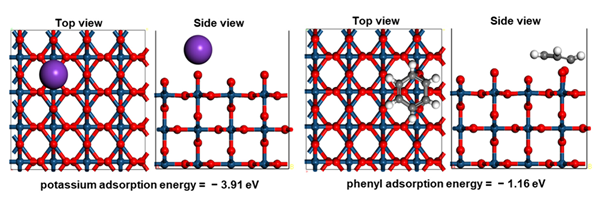 Figure 2. Adsorption energy comparison of potassium ion (left) and benzene (right) on IrO2(110) surface.
Figure 2. Adsorption energy comparison of potassium ion (left) and benzene (right) on IrO2(110) surface.
By adsorbing cationic materials onto the catalyst surface, the direct contact between the ionomer and the catalyst is reduced. This mechanism was validated using density functional theory (DFT), which calculates the electronic structure of materials, thereby demonstrating that ionomer oxidation can be prevented while maintaining hydrogen production performance.
While previous attempts to enhance performance using aqueous potassium hydroxide and sodium hydroxide solutions with high basicity have not elucidated the underlying principles, the competitive adsorption strategy identified in this study holds promise for advancing the commercialization of low-cost catalysts.
Researcher Jihoon Lim, the first author, stated, “The competitive adsorption strategy effectively reduces the electrochemical oxidation of ionomer materials at the interface with the catalyst.”
Professor Lee remarked, “This study sets a path for improving the performance and stability of various energy devices, including high-performance alkaline AEMWE systems.”
The findings were published online in ACS Energy Letters on June 2, 2024. This research was a collaborative effort with Dr. Yu Seung Kim and his team at Los Alamos National Laboratory (LANL) in Los Alamos, New Mexico and Professor Shannon Boettcher at the University of California, Berkeley and the Lawrence Berkeley National Laboratory in Berkeley, California, United States, with support from the U.S. Department of Energy and the National Research Foundation of Korea (NRF).
Journal Reference
Jihoon Lim, Jeffrey M. Klein, Seung Geol Lee, et al., “Addressing the Challenge of Electrochemical Ionomer Oxidation in Future Anion Exchange Membrane Water Electrolyzers,” ACS Energy Lett., (2024).












