A research team, led by Professor Jong-Beom Baek and his team in the School of Energy and Chemical Engineering at UNIST have achieved a significant breakthrough in battery technology. They have developed an innovative method that enables the safe synthesis of fluorinated carbon materials (FCMs) using polytetrafluoroethylene (PTFE) and graphite.
Fluorinated carbon materials have garnered considerable attention due to their exceptional stability, attributed to the strong C-F bonding—the strongest among carbon single bonds. However, traditional methods of fluorination involve highly toxic reagents such as hydrofluoric acid (HF), making them unsuitable for practical applications.
In this study, the research team introduced a straightforward and relatively safe approach for scalable synthesis of FCMs through mechanochemical depolymerization of PTFE—a commonly used compound found in everyday items—and fragmentation of graphite. By utilizing ball-milling techniques that induce both mechanical and chemical reactions, they successfully produced FCMs with significantly improved performance compared to graphite.
The use of hazardous compounds like fluorine gas or HF in conventional carbon fluoride production raises safety concerns, increasing manufacturing costs associated with stringent safety measures. To address these challenges, Professor Baek’s team devised a solid-phase fluorination method using PTFE—an inert polymer known for its stability under atmospheric conditions and harmlessness when consumed orally.
Through experiments, it was observed that subjecting PTFE to higher energy than it can withstand leads to molecular chain breakage and radical formation—initiating a reaction resulting in the production of carbon fluoride complexes. These complexes then adhere to the surface and edges of graphite particles during subsequent processes.
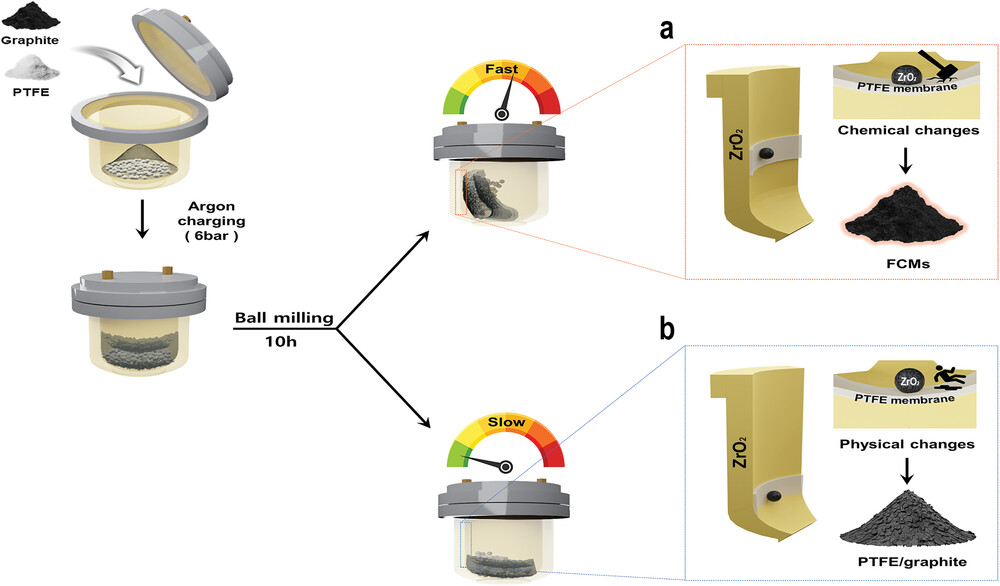
Figure 1. Schematic presentation of mechanochemical ball-milling polytetrafluoroethyelene (PTFE) and graphite at different rotation speeds. a) At the high rotation speed of 450 rpm, chemical changes, such as the depolymerization of PTFE and fragmentation of graphite, were induced to produce FCMs. b) At the low rotation speed of 200 rpm, physical changes, coating, and compounding of PTFE and graphite occurred, to yield PTFE/graphite.
The resulting FCMs demonstrated superior storage capacity and electrochemical stability compared to traditional graphite anodes. At a low charging rate of 50 mA/g, the FCMs exhibited storage capacities 2.5 times higher (951.6 mAh/g) than graphite, while at a high charging rate of 10,000 mA/g, their storage capacity was tenfold higher (329 mAh/g). Remarkably, even after more than 1,000 charge/discharge cycles at a rate of 2,000 mA/g, the FCMs retained 76.6% of their initial capacity compared to only 43.8% for graphite.
“This study highlights not just safe fluorination methods but also the broader potential of solid-phase reactions,” stated Boo-Jae Jang, a researcher in the School of Energy and Chemical Engineering at UNIST.
“This research prompts us to reconsider materials that are commonly found in our surroundings,” added Professor Baek. He further emphasized the significance of understanding solid-phase reactions as it opens doors to developing novel materials that were previously unexplored.
The study findings have been published ahead of their official publication in the online version of Advanced Functional Materials on July 27, 2023. This work has been supported through the U-K Brand and Carbon Neutrality projects of UNIST, and the Creative Research Initiative program through the National Research Foundation (NRF) of Korea.
Journal Reference
Boo-Jae Jang, Qiannan Zhao, Jae-Hoon Baek, et al., “Direct Synthesis of Fluorinated Carbon Materials via a Solid-State Mechanochemical Reaction Between Graphite and PTFE,” Adv. Funct. Mater., (2023).