Cells dispose of their wastes products through the waste removal and recycling system, known as autophagy. This method takes place inside cellular vesicles, called lysosomes.
A joint research team, affiliated with UNIST, has identified the important principle that elucidates the structure of the protein complex plays an important role in selecting which substances to break down and transfers them to the lysosomes. This breakthrough has been led by Professor Changwook Lee in the Department of Biological Sciences at UNIST in collaboration with Professor Youngsoo Jun (School of Life Sciences) from the Cell Logistics Research Center at GIST.
In the study, the research team unveiled that autophagy is selectively regulated through the protein quaternary structure. Published in the September 2019 issue of Autophagy, their findings are expected to provide a new direction for the study of autophagy related diseases.
Various proteins are involved in the collection and transport of cellular waste to lysosomes. Vac8 (Vacuole related 8) is a well-known protein that plays pivotal roles in various autophagic pathways. For example, the Piecemeal microautophagy of the nucleus (PMN) occurs at the nucleus-vacuole junction (NVJ), a well-characterized membrane contact site. On the contrary, when Vac8 binds to the Atg13 (Autophagy related 13) protein, the cytoplasm-to-vacuole targeting (Cvt) pathway, which transports cytoplasmic proteins to the vacuolar lumen in a manner similar to selective autophagy, is being activated. However, the specific principle of how Vac8 binds to these proteins is unknown.
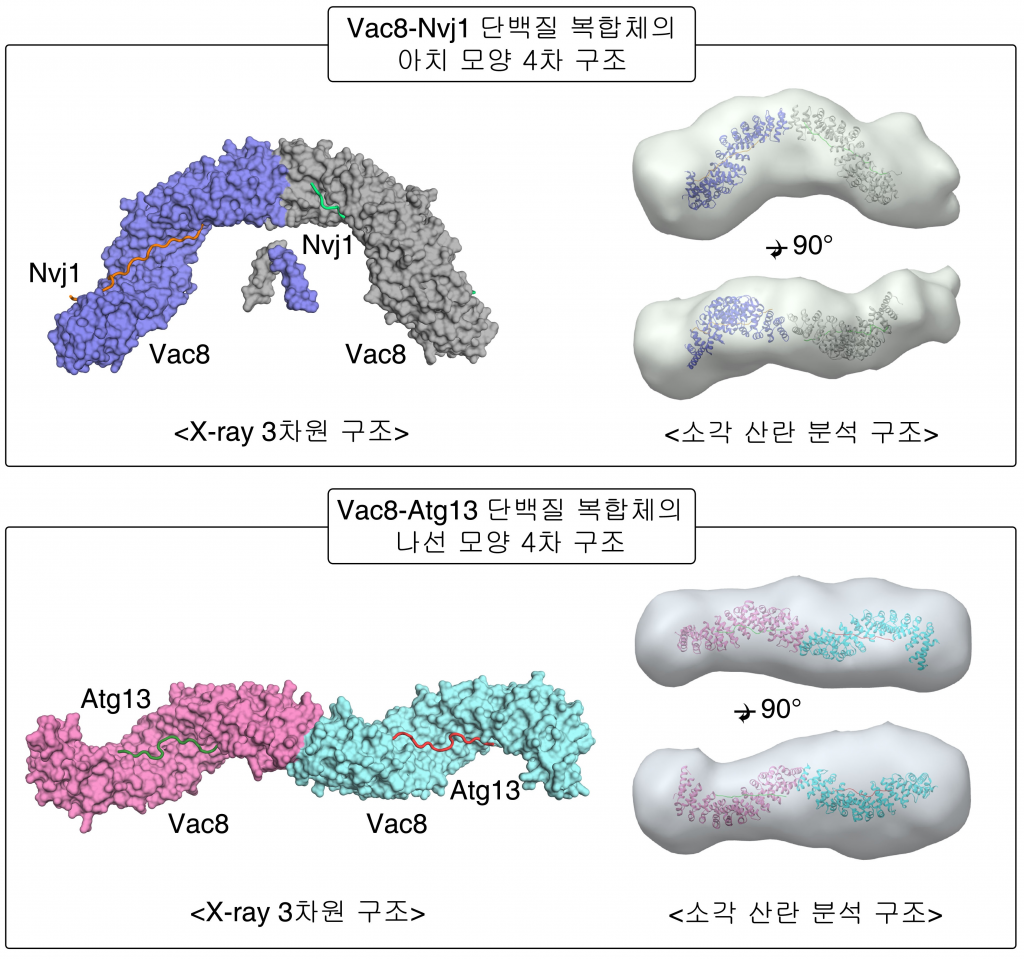
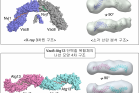
Figure 1. Surface representation of the tVac8-tNvj1 (top) and Vac8[Δ19–33]-Atg13 (bottom) heterotetrameric structures.
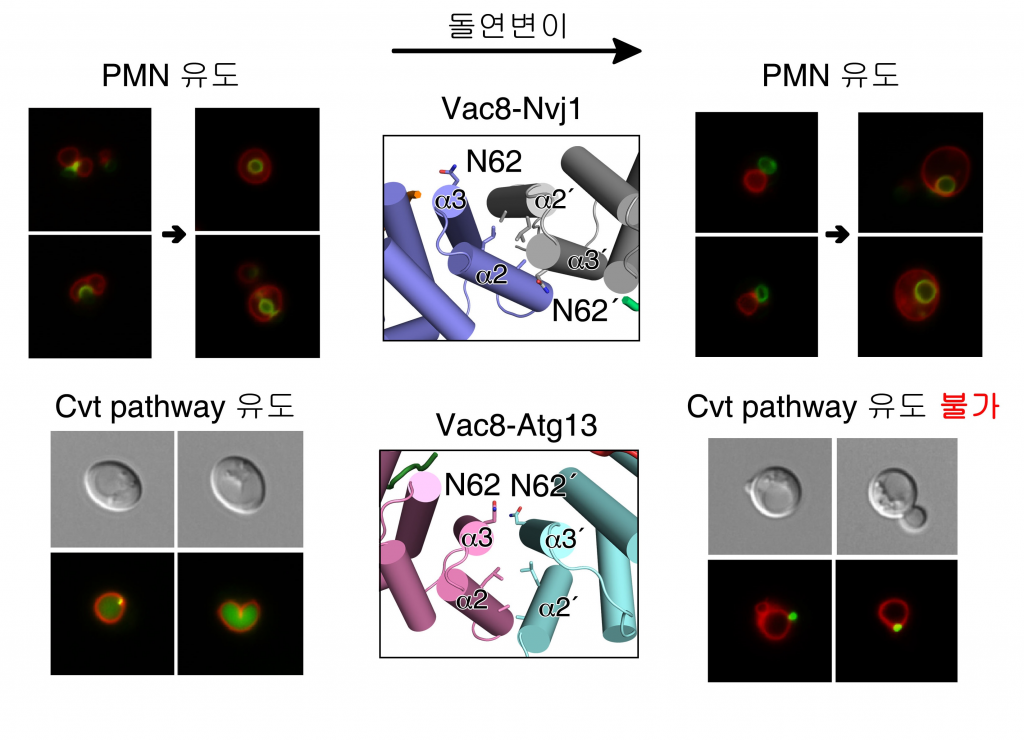
The team also used yeast to induce amino acid mutations involved in Atg13 binding. As a result, PMN autophagy appeared when there was a problem in Atg13 binding structure, but no Cvt related reaction. Abnormalities in the quaternary structure of the protein demonstrated that no specific autophagy occurred.
“This research identifies how a single protein can selectively mediate various forms of autophagy,” notes the research team. “The protein quaternary structure is expected to provide a new direction in discovering treatments for autophagy-related diseases such as Parkinson’s disease, dementia, cancer, and aging.”
The findings of this research have been supported by the Cell Logistics Research Center funded by the National Research Foundation of Korea and by the GIST Research Institute, and it was published on September 12, 2019, in Autophagy (IF = 11.1), which is the leading journal for autophagy research.
Journal Reference
Jumi Park et al., “Quaternary structures of Vac8 differentially regulate the Cvt and PMN pathways,” Autophagy, (2019).