A team of researchers from UNIST has developed a new technology that uses solar energy to produce ammonia and glycolic acid—an essential component in cosmetics—while eliminating carbon dioxide emissions.
Jointly led by Professors Seungho Cho and Myoung Hoon Song in the Department of Materials Science and Engineering at UNIST, the team created a method that converts nitrate pollutants found in wastewater into ammonia through an electrochemical reaction. This process also generates glycolic acid from plastic waste, effectively turning waste into valuable products while reducing carbon emissions.
Ammonia is the second most produced inorganic compound globally, trailing only sulfuric acid, and its production accounts for 1.4% of total carbon dioxide emissions. This underscores the urgent need for eco-friendly alternatives to the traditional Haber-Bosch process.
The research team developed a photovoltaic-electrocatalytic system that produces ammonia at the cathode and glycolic acid at the anode using solar energy. In this system, nitrite (NO₂⁻) in wastewater is reduced at the anode, while ethylene glycol, extracted from plastic waste, is oxidized to glycolic acid at the cathode. Because nitrates (NO₃⁻) and nitrites coexist in wastewater, converting nitrite to ammonia requires significantly less energy and time compared to nitrate, optimizing overall efficiency.
The system achieved an impressive energy efficiency of 52.3%, the highest reported based on anode performance alone, and the ammonia production rate reached 146 μmol/cm²h—well above the U.S. Department of Energy’s commercialization criterion of 58.72 μmol/cm²h, marking a 46% improvement over previous records.
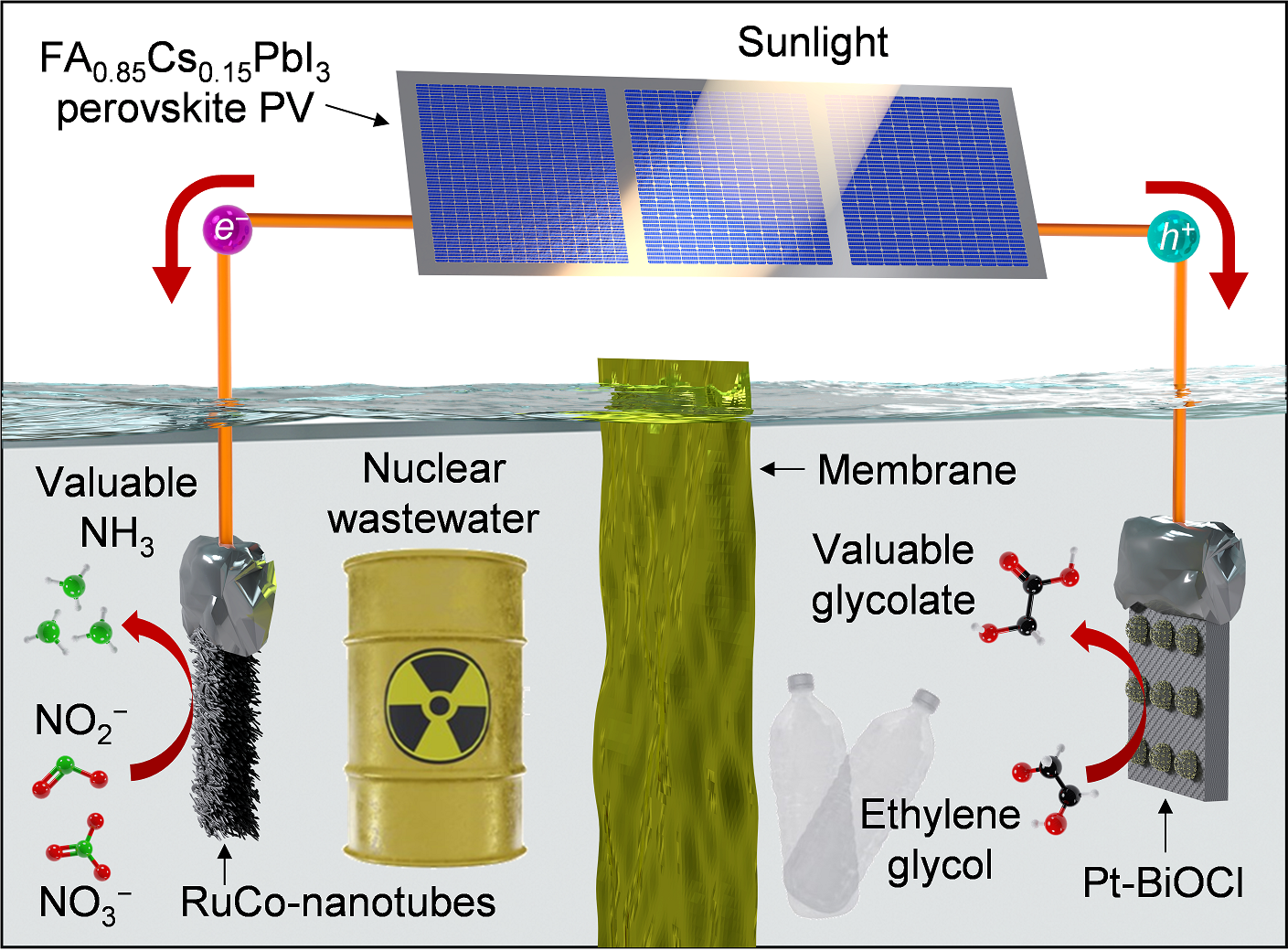 Figure 1. Schematic representation illustrating the key findings of the study.
Figure 1. Schematic representation illustrating the key findings of the study.
The researchers developed an efficient catalyst (RuCo-NT/CF) that selectively reduces nitrite, further enhancing the system’s effectiveness. By specifically targeting nitrite and avoiding the energy-intensive oxygen evolution reaction, they fine-tuned the process for better results.
The perovskite solar cell used in the system was designed for high electrical output and durability. As the photovoltaic current density increases, so does the rate of ammonia production.
Professor Song noted, “This research highlights the potential for carbon dioxide-free electrochemical ammonia production using perovskite solar cells, which outperform commercial silicon solar cells.”
The team assessed the technology’s commercialization potential, finding that their electrochemical system could achieve a solar ammonia production rate of 114 μmol/cm²h when using an electrolyte that simulates low-level radioactive wastewater and extracted PET materials.
Professor Cho added, “Our study offers a sustainable, carbon-neutral energy solution by simultaneously producing green ammonia and glycolic acid from sunlight and waste.”
This research involved joint first authors Wonsik Jang, Jongkyoung Kim, and Hye Seung Kim and received support from the Basic Research Laboratory Program of the Ministry of Science and ICT (MSIT). The findings were published in Nano Letters on February 19, 2025.
Journal Reference
Wonsik Jang, Jongkyoung Kim, Hye Seung Kim, et al., “Solar-Driven High-Rate Ammonia Production from Wastewater Coupled with Plastic Waste Reforming,” Nano Lett., (2025).