Biomarkers are components that may be present in biological samples and are related to specific diseases. Therefore, doctors can analyze biological samples from a patient to check their health condition or to monitor the progress of a specific therapy. Typically, these samples need to be purified and diluted before the analysis, and current medical diagnostic techniques rely on healthcare facilities and laboratories for these routine analyses. This is a lengthy process that requires trained personnel and expensive instrumentation to extract, transport, store, process, and analyze the samples in centralized locations. Moreover, during a period of global crisis like the ongoing pandemic, the pressure of thousands of analysis requests can saturate and collapse the healthcare system.
On the other hand, point-of-care devices, which are small automated instruments, are capable of performing diagnostics in decentralized locations and can provide quick answers. One example of such a device is the glucose meter that people with diabetes use to monitor their sugar levels in the blood. These devices can overcome the inherent limitations of having to process a sample through a centralized system, empowering anyone to be able to monitor their health from home, simply using a tiny blood sample extracted with a fingerprick.
However, the development of these devices has been burdened by the technical challenges related to measuring biological samples. Biomarkers for some diseases and infections are only present in the samples in very small amounts, which in turn imposes the challenge to develop extremely sensitive detection techniques. While increasing the surface area of the biosensor can increase the sensitivity of the instrument, these surfaces tend to be quickly clogged and contaminated, rendering them unusable.
To this end, Professor Yoon-Kyoung Cho (Department of Biomedical Engineering) and her research team from the Center for Soft and Living Matter (CSLM), within the Institute for Basic Science (IBS) at UNIST recently developed a biosensor using a method to generate nanostructured and nanoporous surfaces. This combined strategy not only provides the sensor with an unprecedented sensitivity but also makes it resistant to fouling by proteins.
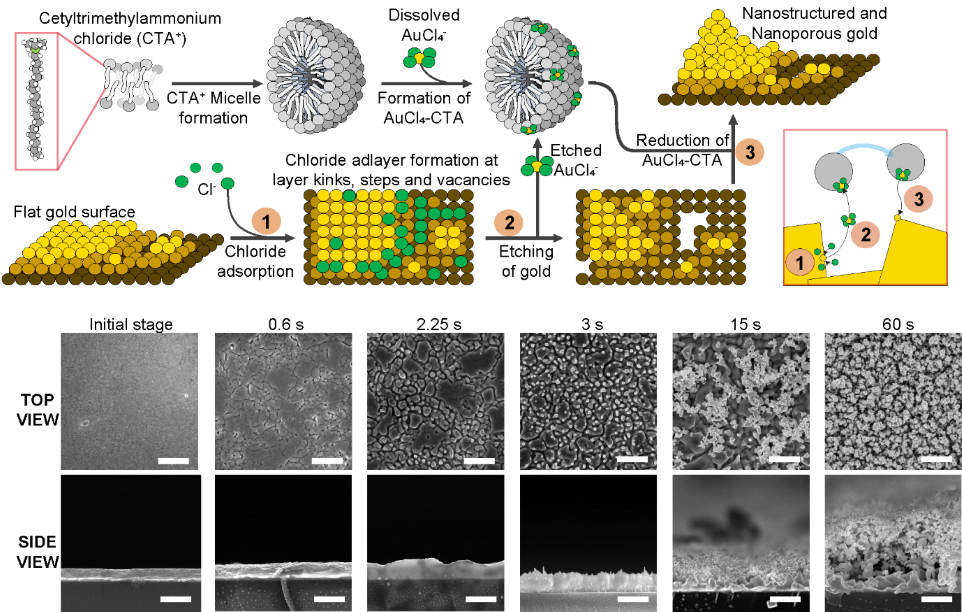
Figure 1. Mechanism to generate nanostructured and nanoporous gold surfaces based on the preferential etch and deposition of the substrate using a surfactant that forms micelles in solution, sodium chloride, and a gold salt. Applying electric pulses, first, chloride is adsorbed on the surface, then gold is etched away but captured by the surfactant micelles. Finally, it is redeposited on the substrate growing the nanostructures in the process. At the bottom, scanning electron micrographs show the formation of nanostructures and nanopores on the surface throughout the process.
While previously there has been no known method to reliably create electrodes using such nanostructured and nanoporous substrates, the team reported a simple method to generate such materials. The mechanism is based on the application of electric pulses to a flat gold surface in the presence of sodium chloride and a surfactant that can form micelles in solution. These electric pulses drive a preferent reaction to etch and redeposit gold from the surface and, in turn, grow nanostructures and form the nanopores (See Figure 1). The use of surfactant in the form of micelles is essential to the success of this strategy since it prevents the material that is being etched from diffusing away during the process, so it can be redeposited.
The formation of these nanostructures yielded a large surface area which was beneficial for increasing the sensitivity of the assays, whereas the formation of nanopore substrates was ideal to prevent contamination from the biological samples. Both the nanostructures and the nanopores’ combined benefits were key to the success of this strategy, which could be applied for the direct analysis of clinical plasma samples.
The researchers further demonstrated this new technology by building a biosensor for the detection of prostate cancer. The electrode was sensitive enough to discriminate between a group of prostate cancer and healthy donors using only a tiny amount of blood plasma or urine samples. No dilution or preprocessing steps were used, which means that the technology could easily be used for the point-of-care diagnosis of cancer.
“We believe that this technology is essential for the future development of point-of-care devices and diagnostic tests that work with biological samples,” said Professor Cho. “The capability to detect low concentrations of relevant biomarkers with robust performance opens a door to possibilities in the field of diagnostics for cancer, pathogens, and other diseases.”
The findings of this research have been published in the journal, Advanced Materials (IF: 30.849) on May 17, 2022. The associated illustration has also been selected for the frontispiece in the current issue.
Yoon-Kyoung Cho
Professor, School of Life Sciences, UNIST
IBS Center for Soft and Living Matter
William I. Suh
IBS Public Relations Team
T: +82-42-878-8137
E:willisuh@ibs.re.kr
Story Source
Materials provided by the Institute of Basic Science.
Notes for Editors
This press release is made available courtesy of IBS Communications Team. The online version of the original article can be found HERE.
Journal Reference
Jonathan Sabaté del Río,Hyun-Kyung Woo,Juhee Park, et. al, “SEEDING to Enable Sensitive Electrochemical Detection of Biomarkers in Undiluted Biological Samples,” Advanced Materials, (2022). DOI: 10.1002/adma.202200981














