A research team, affiliated with UNIST has reported that they have established the molecular mechanism via which MIGA2 (Mitoguardin2) traffics glycerophospholipids between membranes of the ER and mitochondria.
This breakthrough has been carried out by Professor Changwook Lee and his research team in the Department of Biological Sciences at UNIST. According to the research team, the structural information and biochemical experiments reported in this paper provide a framework to understand the molecular mechanism by which MIGA2 mediates the formation of ER-mitochondria contact sites and facilitates lipid trafficking between anchored membranes in mammalian cells.
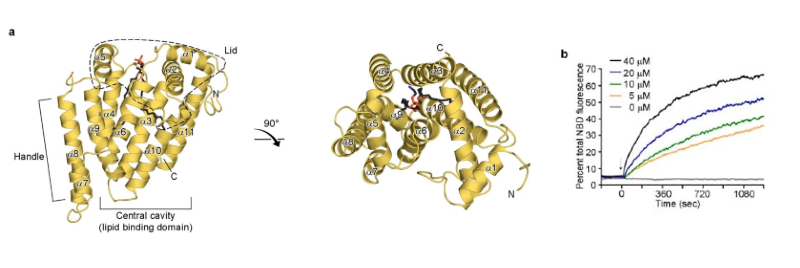
Figure 1. Overall structure of zMiga2. a) Ribbon representation of the zMiga2 (yellow orange). The structure was determined by Se-SAD phasing and refined to 2.85 Å resolution. The overall structure is reminiscent of a mug. The lid is highlighted by dashed lines. The handle protrudes on the left. The black stick model indicates the phospholipid bound to zMiga2. Phosphate, nitrogen, and oxygen atoms are colored in orange, blue, and red, respectively. b) In vitro lipid transfer assay. The graph shows time courses for fluorescence emitted by NBD-PE in concentration-dependent reactions. The fluorescence output of NBD-PE transferred from the donor liposome (50 µM) to the acceptor liposome (50 µM) was monitored upon addition of various concentrations of zMiga2. The arrow indicates the point of protein injection.
The findings of this research have been published in the June 2022 issue of Nature Communications, an open access scientific journal published by Springer Nature. This research has been supported by the grants from the National Research Foundation of Korea.
Journal Reference
Hyunwoo Kim, Seowhang Lee, Youngsoo Jun et al., “Structural basis for mitoguardin-2 mediated lipid transport at ER-mitochondrial membrane contact sites,” Nat. Commun., (2022).