Water, which makes up more than half of the human body, is an essential participant in the structure and function of proteins. Hormones and enzymes are also proteins, and thus are responsible for the biological function of the human body. Therefore, understanding the unique properties of water helps identify the cause of disease and accelerate drug development. A novel technology has recently been announced to make this possible.
A research team, led by Professor Oh-Hoon Kwon in the School of Natural Sciences at UNIST has proposed a new experimental approach for estimating the H‐bond free energy of local biological water. This was made possible by combining time‐resolved spectroscopy and installation of a fluorescent, prototropic hydration probe at protein surfaces. This study has been jointly conducted by Professor Sang Kyu Kwak (School of Energy and Chemical Engineering, UNIST), and Professor Tae Hyeon Yoo (Department of Molecular Science and Technology, Ajou University).
A hydrogen bond is a type of attractive intermolecular force that exists between atoms with different electrical charges. This bond plays a significant role in determining the intermolecular bonding between water molecules, as well as the energetics and structures of biopolymers. In addition to this, H-bonds are responsible for the formation of biopolymer structures indirectly since they determine the water structure. Therefore, in order to better understand the energetics and structures of biopolymers, it is necessary to study the occurrence of H-bonding in water molecules at a particular location. However, water molecules surrounding these biopolymers further add to the complexity of the analysis of H-bond free energy of water at the desired position.
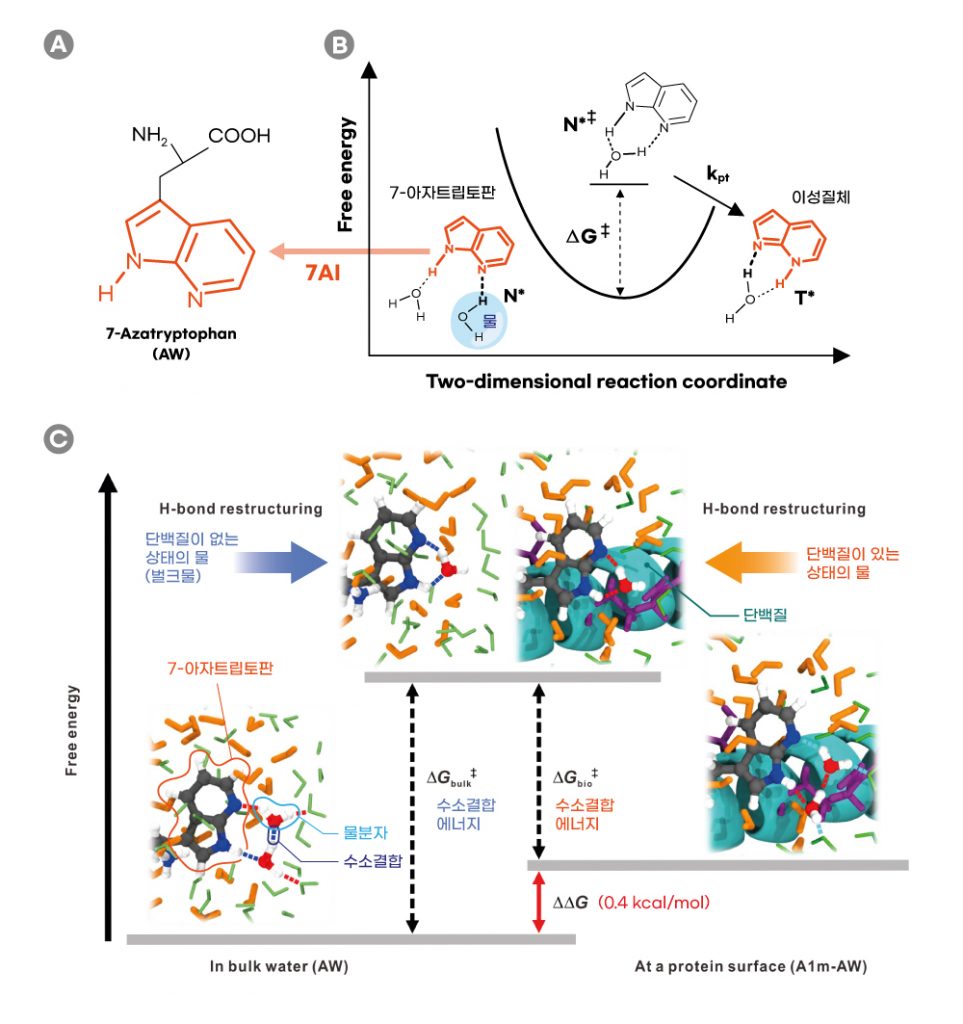
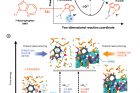
Figure 1. Schematic comparison of the H‐bonding energetics of water in the bulk and in the vicinity of the model protein surface probed by the proton‐transfer reaction to measure the H‐bond free‐energy difference (ΔΔG ) between bulk water and biological water.
In the study, the joint research team proposed an experimental methodology based on femtosecond‐resolved fluorescence spectroscopy to measure the H‐bond free energy of water at protein surfaces under isothermal conditions. A demonstration was conducted by installing a non‐canonical isostere of tryptophan (7‐azatryptophan) at the surface of a coiled‐coil protein to exploit the photoinduced proton transfer of its chromophoric moiety, 7‐azaindole.
In the excited state, this 7‐azatryptophan takes hydrogen ions (H⁺) from neighboring water molecules, then gives them back to the water molecules. In this process, the H-bond found between water molecules is broken, and then rearranged. Therefore, the stronger the bond, the more energy it takes to break the bond, and hence, ultimately slows down the reaction.
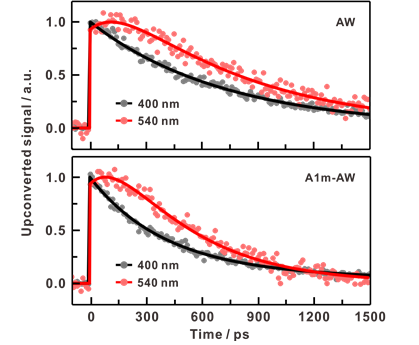
Figure 2. Fluorescence kinetic profiles of AW and A1m‐AW representing excited‐state proton transfer. The excitation wavelength was 285 nm. a) Time‐resolved fluorescence of AW (upper panel) and A1m‐AW (lower panel) measured at 400 nm (black) and 540 nm (red). The profiles were measured at 400 nm to trace the temporal dependence of [N*] and at 540 nm to trace [T*].
The researchers evaluated the H‐bond free energy of this biological water by comparing the rates of proton transfer, sensitive to the hydration environment, at the protein surface, and in bulk water. The results of experiments and simulations utilizing computational methodologies show that the H‐bond free energy of biological water in this study was higher than that of bulk water. According to the research team, “The difference may be due to the entropic cost of the highly structured H‐bond network in the vicinity of the hydrophilic and charged protein surfaces.”
“This was made possible by combining time‐resolved spectroscopy and installation of a fluorescent, prototropic hydration probe at protein surfaces,” says Won-Woo Park (Combined M.S/Ph.D. of Energy and Chemical Engineering, UNIST), the first author of the study. “Thus, using the pump-probe technique, we were able to track the well‐established photoinduced prototropy of the fluorescent residue of 7‐azatryptophan at protein surfaces and in bulk water.”
“Our study opens a door to accessing the energetics and dynamics of local biological water to give insight into its roles in protein structure and function,” says Professor Kwon. “Furthermore, it can be used to help researchers understand the role of water in biological assemblies and biochemical processes, for example, maintaining and modulating ligand binding.” He adds, “The utility of this approach, if confirmed, would likely accelerate new drug discovery.”
The findings of this research have been published in the
internationally renowned journal, Angewandte Chemie International Edition on April 27, 2020. The publication was highlighted by Angewandte Chemie as “hot paper” and featured as the back cover artwork in the April 2020 edition of the journal. This study has been supported by the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (MSIT), the Ministry of Education, and by the Institute for Basic Science (IBS), Korea.
Journal Reference
Won‐Woo Park, Kyung Min Lee, Dr. Byeong Sung Lee, et al., “Hydrogen‐Bond Free Energy of Local Biological Water,” Angewandte Chemie, (2020).