A research team, jointly led by Professor Hyunchul Oh in the Department of Chemistry at UNIST and Professor Eunsung Lee at Seoul National University, has introduced a novel approach, capable of adjusting the pore size of porous materials in increments of 0.01 nm (10-9 m).
This innovative technology efficiently separates deuterium, an isotope of hydrogen that constitutes only 0.015% of the total hydrogen found in nature and shares similar properties with ordinary hydrogen. Deuterium is a crucial resource for applications in nuclear fusion power generation and semiconductor manufacturing, making its efficient separation highly valuable.
The research team demonstrated that the pore sizes of metal-organic frameworks (MOFs) can be fine-tuned through ion exchange, achieving unprecedented control and significantly enhancing the separation efficiency of deuterium in these materials.
To optimize separation efficiency, the researchers illustrated that deuterium and hydrogen can effectively be separated using the precisely adjusted pores of a newly developed MOF. It is essential to finely calibrate the pore size, which acts as a molecular sieve. Given that both hydrogen and deuterium are approximately 0.3 nm in size, achieving precise modifications at the 0.01 nm scale is critical.
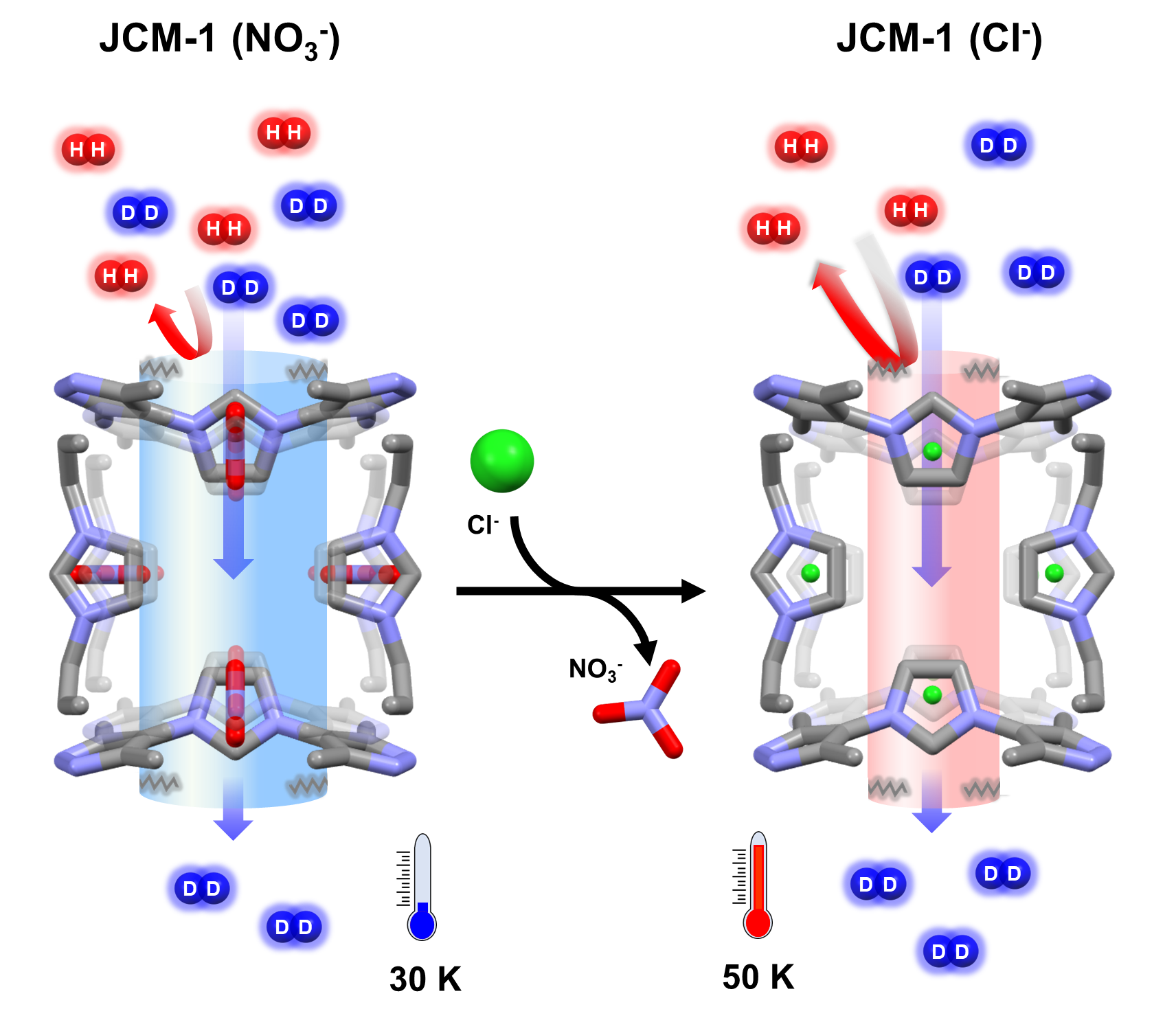 Figure 1. A schematic diagram representing the summary of the study. Gate-opening control via anion exchange in JCM-1 enables enhanced hydrogen isotope separation through precise tuning of pore aperture and diffusion barriers. The dynamic interplay between structural flexibility and adsorption energetics maximizes D2/H2 selectivity, establishing a robust platform for cryogenic gas separation applications.
Figure 1. A schematic diagram representing the summary of the study. Gate-opening control via anion exchange in JCM-1 enables enhanced hydrogen isotope separation through precise tuning of pore aperture and diffusion barriers. The dynamic interplay between structural flexibility and adsorption energetics maximizes D2/H2 selectivity, establishing a robust platform for cryogenic gas separation applications.
The researchers successfully reduced the pore inlet size of the JCM-1 MOF from about 0.39 nanometers to 0.36 nanometers by replacing nitrate ions (NO₃⁻) with chloride ions (Cl⁻). Their analysis revealed that Cl⁻ induces a stronger inward pull on the outer framework connected to the pore, resulting in a smaller pore size.
As a result of this modification, the selectivity for deuterium separation in JCM-1 (Cl⁻) increased significantly from 14.4 to 27.7, effectively doubling the efficiency compared to JCM-1 (NO₃⁻). Notably, JCM-1 (NO₃⁻) exhibited selectivity nine times greater than the conventional cryogenic distillation method at 24K (-249.15°C), while JCM-1 (Cl⁻) demonstrated over 18 times higher selectivity than that method.
Professor Oh, the lead investigator, stated, “This achievement offers a novel approach to precisely controlling the nanopore sizes of porous materials, with potential applications not only in isotope separation but also in various gas separation processes.”
Their findings have been published in Angewandte Chemie International Edition (Impact Factor: 16.1) on February 12, 2025. This research was funded by the National Research Foundation of Korea (NRF) and the Ministry of Science and ICT (MSIT).
Journal Reference
Hyunlim Kim, Younggyu Seo, Jaewoo Park, et al., “A Gate-Opening Control Strategy via Nitrate–Chloride Anion Exchange for Enhanced Hydrogen Isotope Separation in Metal–Organic Frameworks,” Angew. Chem. Int. Ed., (2025).