A research team, affiliated with UNIST has achieved a groundbreaking breakthrough in the field of electrocatalysis. In this study, they have succeeded in demonstrating a facile approach to realizing heavily doped molybdenum disulfide (MoS2) with a high doping concentration exceeding 16%, using intermediate-reaction-mediated chemical vapor deposition (CVD).
Jointly led by Professor Hyesung Park (Department of Materials Science and Engineering, UNIST), Professor Young-Kyu Han from Dongguk University-Seoul, and Professor Jeong Min Baik from Sungkyunkwan University (SKKU), the study sheds light on the controllable synthesis of highly doped transition metal dichalcogenides (TMDs) and their impact on catalytic activity.
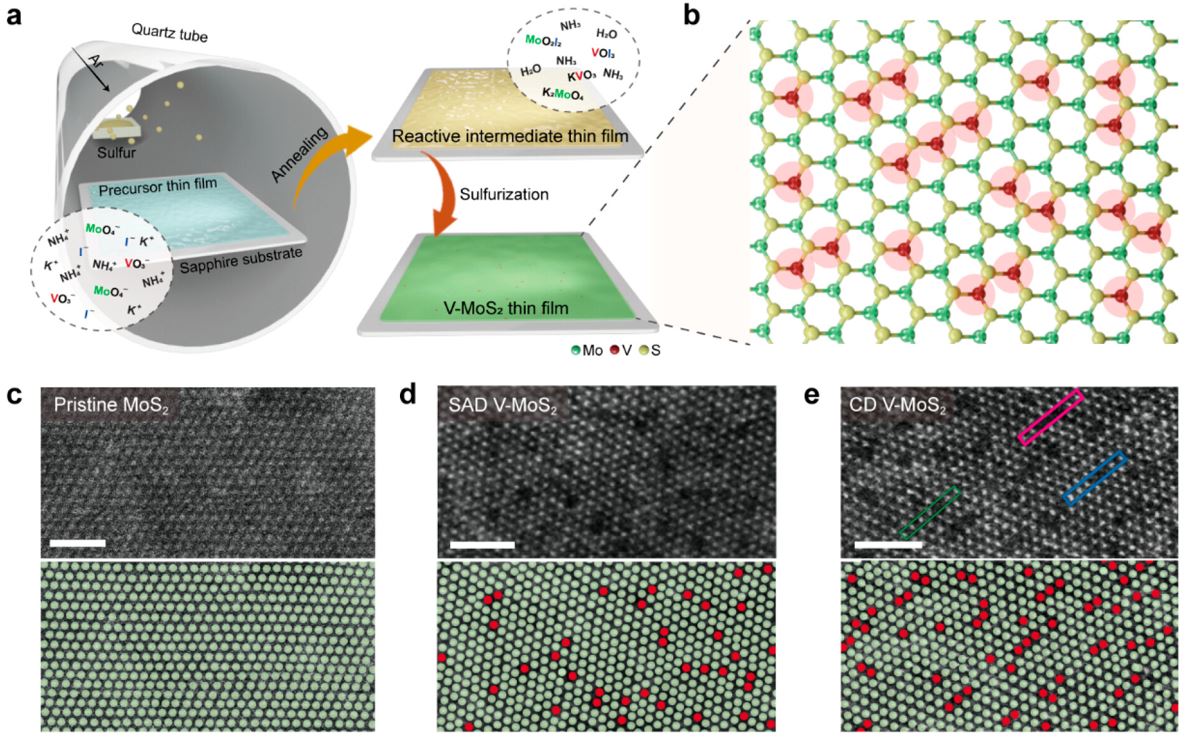
Figure 1. Characterization of substitutional V doping in MoS2. (a) Schematic of V-MoS2 thin film growth by intermediate-reaction-mediated liquid-precursor CVD and (b) atomic lattice structure of as-synthesized V-MoS2. (c–e) HAADF-STEM image of pristine MoS2, SAD V-MoS2, and CD V-MoS2 (scale bars: 2 nm). Mo and V atoms are marked by green and red circles, respectively.
The introduction of heteroatoms has long been recognized as an effective strategy to enhance the intrinsic catalytic activity of TMDs. By activating the otherwise inactive basal plane, this approach boosts catalytic performance. However, until now, understanding the influence of atomic configurations within TMD lattices on catalytic activity has remained limited due to a lack of controllable synthetic methods for achieving highly doped TMDs.
Addressing this gap in knowledge, researchers successfully realized heavily doped MoS2 with exceptional doping concentrations above 16% using intermediate-reaction-mediated chemical vapor deposition. As the concentration of vanadium (V) dopants increased, these incorporated atoms exhibited coalescence behavior that activated both the basal plane and enhanced electrical conductivity within MoS2.
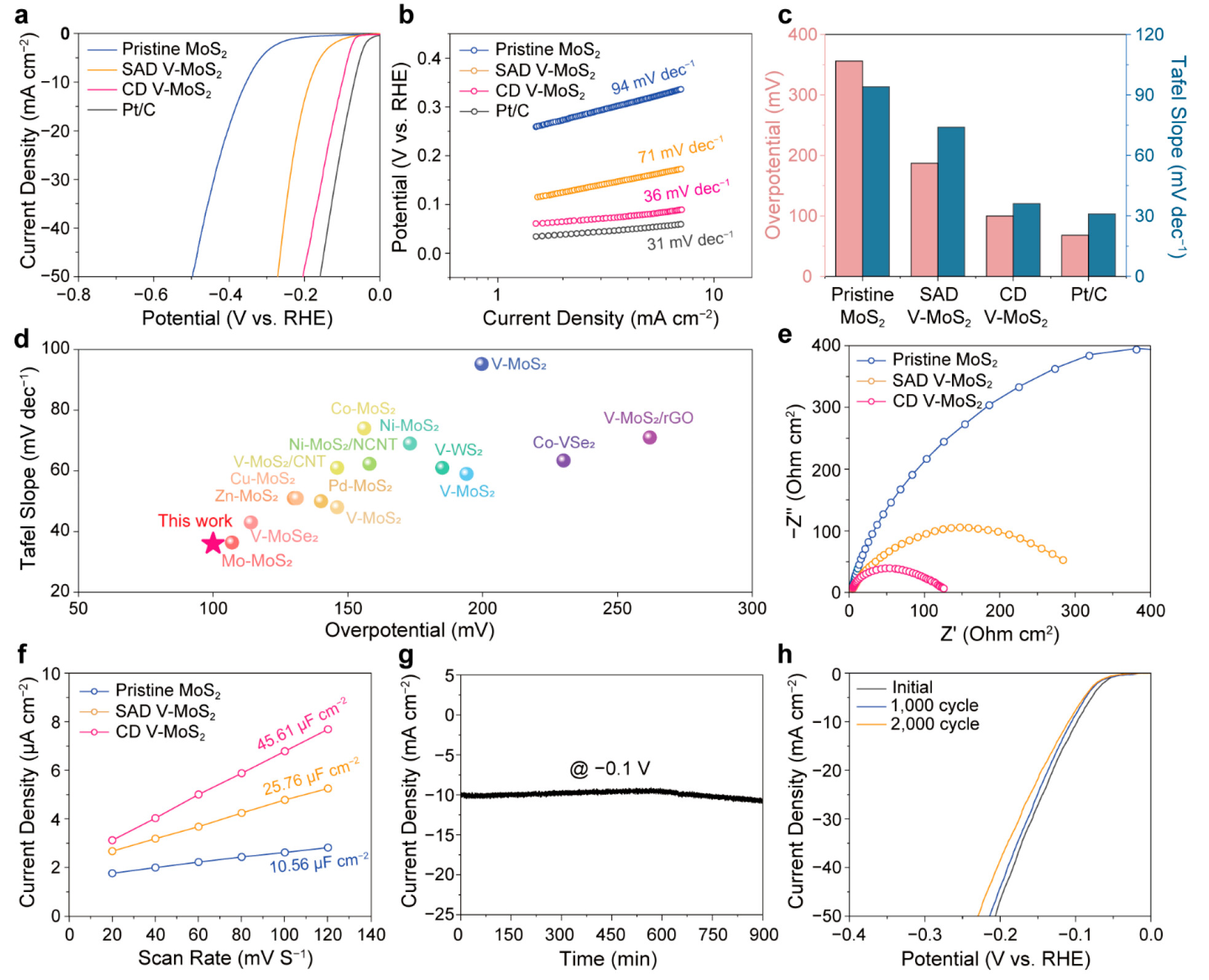
Figure 2. Electrocatalytic HER performance of V-MoS2. (a) LSV polarization curve, (b) Tafel plot, and (c) summary of the overpotential values at 10 mA cm–2 and Tafel slopes of pristine MoS2, SAD V-MoS2, CD V-MoS2, and Pt/C. (d) Comparison of CD V-MoS2 HER performance with state-of-the-art transition metal doped TMD-based electrocatalysts. The detailed catalyst information is presented in Table S1. (e) Nyquist plot and (f) electrochemical double-layer capacitance for pristine MoS2, SAD V-MoS2, and CD V-MoS2. (g) Time-dependent current density of CD V-MoS2 at a static potential of −0.1 V for 900 min. (h) LSV curve of CD V-MoS2 before and after 1000 and 2000 cycle tests.
Experimental and theoretical analyses confirmed that this coalesced V-doping configuration significantly accelerated kinetics during hydrogen evolution reaction (HER), resulting in reduced Gibbs free energy for hydrogen adsorption. Consequently, the coalesced V-doped MoS2 exhibited outstanding HER performance compared to pristine and single-atom-doped counterparts. It demonstrated an overpotential as low as 100 mV at 10 mA cm–2.
These findings unlock intriguing possibilities for engineering atomic doping configurations within transition metal dichalcogenides (TMDs), paving the way for efficient electrocatalysts based on two-dimensional (2D) nanomaterials. The ability to precisely control doping concentrations and arrangements within TMDs holds immense promise for advancing electrocatalysis across various fields.
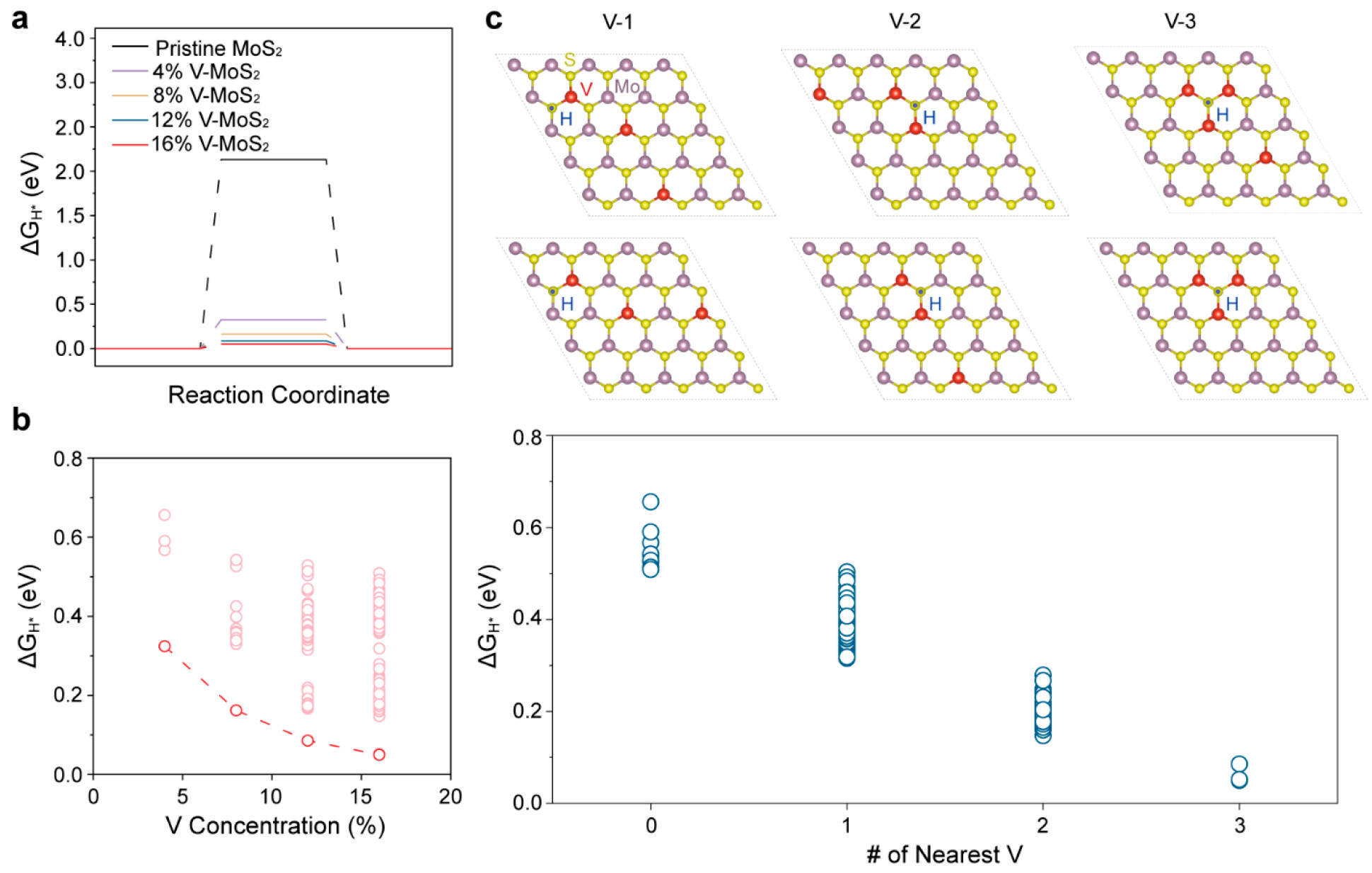
Figure 3. Doping concentration and configuration-dependent HER performance of V-MoS2. (a) Free energy diagram for HER on MoS2 with different V doping concentrations. (b) Free energy for HER with respect to the V concentrations for all available hydrogen adsorption sites near V dopants. (c) Example structure for V-MoS2 (V-1, V-2, and V-3) and free energy for HER with respect to the number of V atoms nearest to the adsorption site.
“This breakthrough holds immense promise for various applications requiring enhanced catalytic activity, particularly in energy conversion and storage systems,” noted the research team. Their work paves the way for target-oriented platforms, enabling scientists to design highly efficient 2D nanomaterial-based electrocatalysts tailored to specific needs.
The study findings have been published ahead of their official publication in the online version of ACS Nano on May 15, 2023. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Korea government (MSIT).
Journal Reference
Eunbin Son, Sangjin Lee, Jihyung Seo, et al., “Engineering the Local Atomic Configuration in 2H TMDs for Efficient Electrocatalytic Hydrogen Evolution,” ACS Nano, (2023)
















