A groundbreaking catalyst technology for the production of sustainable aviation fuel (SAF) has been developed, promising a significant reduction in carbon emissions through the utilization of carbon dioxide. This innovation is expected to contribute to carbon neutrality by capturing CO2 from the atmosphere and converting it into isoparaffins, high-value hydrocarbons.
A research team, led by Professor Kwangjin An in the School of Energy and Chemical Engineering at UNIST, in collaboration with the Carbon Neutrality Research TFT of LG Chem Ltd., has developed a catalyst specifically designed for producing isoparaffins suitable for SAF production from carbon dioxide. This new catalyst has successfully replaced traditional zeolite catalysts when used in conjunction with iron-based systems, resulting in a significant increase in the isoparaffin production rate.
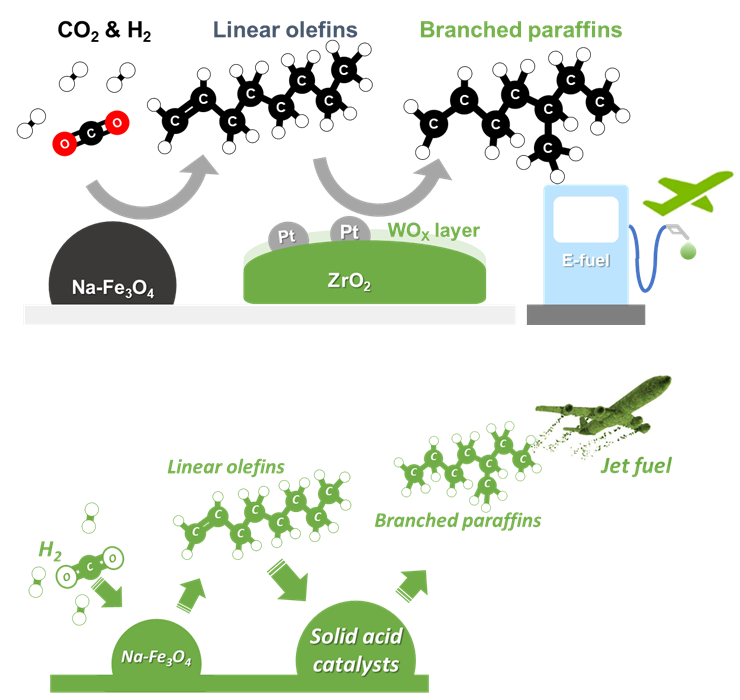 Figure 1. Schematic diagram showing the overall production process of isoparaffin via direct hydrogenation of CO2 using solid acid catalysts.
Figure 1. Schematic diagram showing the overall production process of isoparaffin via direct hydrogenation of CO2 using solid acid catalysts.
Notably, aircraft fuel produced using platinum-based tungsten-zirconia (Pt/WZ) catalysts is anticipated to reduce carbon emissions by up to 80% compared to conventional fossil fuel-based aviation fuel. This innovative process features enhanced efficiency by enabling the direct conversion of carbon dioxide into isoparaffins.
The catalyst shows strong potential for commercialization due to its low carbon deposition and stable reactivity over extended periods. Its applications are expected to benefit not only the aviation industry but also other sectors of transportation in the fight against carbon emissions.
Professor An stated, “We have developed a new catalytic method that addresses the limitations of existing zeolite catalysts, which are prone to coke formation during reactions, thus maximizing the production rate of isoparaffins.” Won-Hee Kim from LG Chem Ltd., the lead researcher, emphasized, “This technology holds significant industrial application value as it can enhance fuel efficiency while minimizing additional costs associated with refining.”
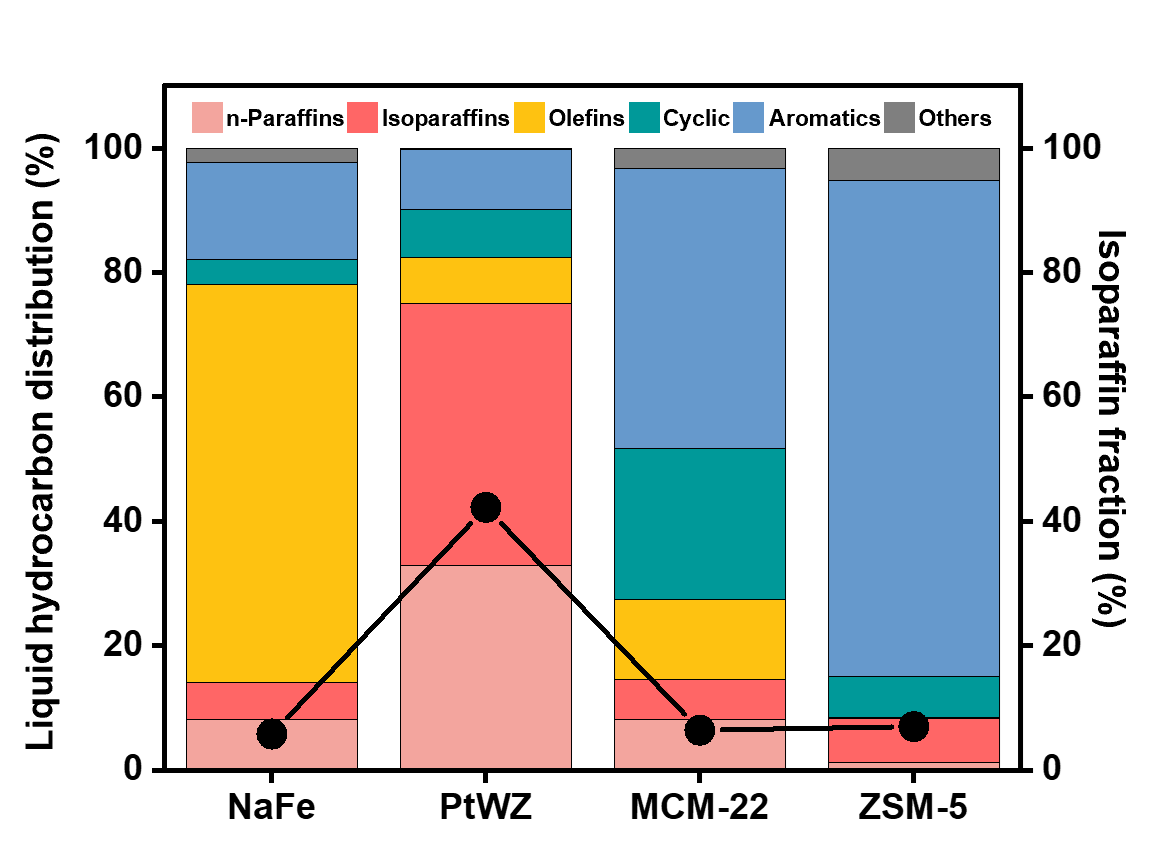 Figure 2. Catalytic performance of various solid acid catalysts used with a NaFe catalyst for CO2 hydrogenation. Liquid hydrocarbon distribution of solid acid catalysts (Y-axis on the left represents the hydrocarbon distribution of the liquid product, and black dots represent the fraction of isoparaffins).
Figure 2. Catalytic performance of various solid acid catalysts used with a NaFe catalyst for CO2 hydrogenation. Liquid hydrocarbon distribution of solid acid catalysts (Y-axis on the left represents the hydrocarbon distribution of the liquid product, and black dots represent the fraction of isoparaffins).
Currently, the air transport sector is responsible for 24.5% of total carbon dioxide emissions, making the commercialization of SAF a pressing challenge for achieving carbon neutrality. The International Air Transport Association (IATA) is intensifying regulations to promote the adoption of SAF.
This research was conducted with technical support from the Korea Basic Science Institute (KBSI) and the findings were published online in ACS Catalysis on August 9, 2024. The study was additionally supported by the National Research Foundation of Korea (NRF) and the Ministry of Trade, Industry and Energy (MOTIE).
Journal Reference
Hojeong Lee, Changhun Hur, Yong Hee Lee, et al., “Enhanced Isoparaffin Selectivity in CO2 Hydrogenation by Combining Na-Promoted Fe3O4 and Pt/WO3-ZrO2 Catalysts,” ACS Catal., (2024).