An international research team, affiliated with UNIST has developed a new electrocatalyst for aqueous Zn–CO2 system, while eliminating carbon the harmful greenhouse gas carbon dioxide (CO2). This is expected to further accelerate the commercialization of the previously reported aqueous Zn or Al–CO2 system.
A joint research team, led by Professor Guntae Kim and Professor Jong Beom Baek in the School of Energy and Chemical Engineering at UNIST has reported a unique approach to fabricate uniformly anchored ruthenium (Ru) nanoparticles on carboxyl-functionalized porous sphere carbon (CF-Ru@PSC) with a strongly bonded interaction between the metallic Ru and PSC substrate for the aqueous Zn–CO2 system. The new electrocatalyst not only works well under the CO2-saturated conditions, but can also bring great potential for commercialization success since it can be synthesized by simple steps, using feasible low-cost materials.
Aqueous Zn–CO2 system utilizes CO2 as a raw material to generate electrical energy and hydrogen (H2). The research team had previously developed this system based on the phenomenon that the ocean contains significantly larger amounts of CO2 dissolved in it. Within this system, the hydrogen ions (H+) that are produced by dissolving CO2 in water are reduced through electrochemical reactions to produce H2.
At this time, a catalyst is used to lower the activation barriers of the electrochemical reactions and precious metal catalysts, such as platinum (Pt) were used for the existing metal–CO2 systems. Various other metal oxides and carbon-based catalysts have been investigated as alternatives to noble metal-based catalysts (e.g., Pt), but some of their major drawbacks include their low hydrogen evolution reaction (HER) activity in CO2 saturated environments.
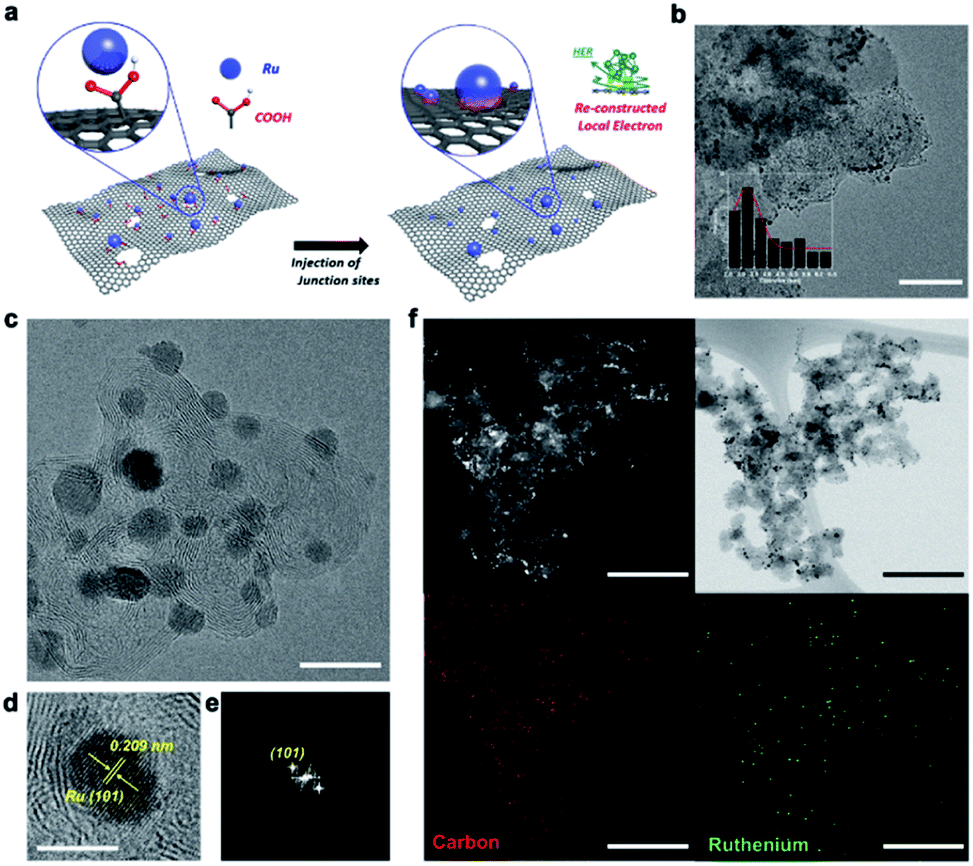
Figure 1. Structural analysis and HR-TEM characterization of the CF-Ru@PSC catalyst. (a) Schematic illustration of the synthesis and structure of the CF-Ru@PSC electrocatalyst. (b) Low-resolution TEM image (scale bar: 50 nm). Inset: corresponding Ru particle distribution. (c) High-resolution TEM image (scale bar: 10 nm) of CF-Ru@PSC. (d and e) Magnified images of CF-Ru@PSC (scale bar: 4 nm): TEM and corresponding FFT pattern. (f) BF and HAADF-STEM (top) and corresponding elemental mapping (bottom) of CF-Ru@PSC. Scale bar: 100 nm.
In this work, the joint research team has developed a metal organic framework catalyst that works well under a CO2 saturated condition. According to the research team, the CF-Ru@PSC catalyst also exhibited an exceptionally high electrochemical performance (45.4 mW cm−2, achieving 107% of that based on the commercial Pt/C) and structural stability over 1000 min of consecutive operation with a remarkably high Faraday efficiency (98.2%) in the aqueous Zn–CO2 system. This phenomenal catalytic performance of the CF-Ru@PSC catalyst is associated with the strongly embedded Ru on the carbon substrate from a one-step removal process of the carboxylic group (–COOH).
“The removal of –COOH functional induces a strong bond between the Ru nanoparticles and PSC substrates, as well as the additional electrochemical effective active sites between them,” says Jeongwon Kim (Combined MS/Ph.D program of Energy and Chemical Engineering, UNIST), the first author of the study.
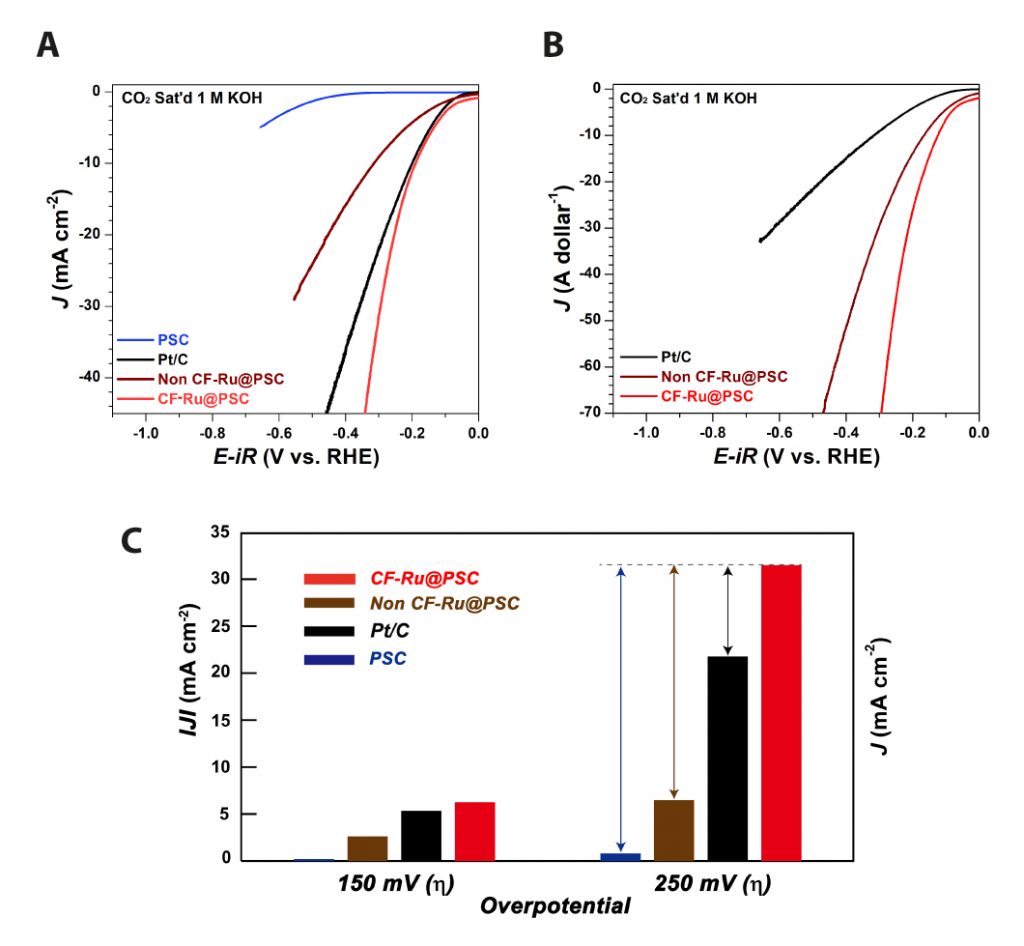
Figure 2. Electrocatalytic HER activity of the CF-Ru@PSC catalyst. (a) Polarization curves and corresponding Tafel plots of PSC, Non CF-Ru@PSC, CF-Ru@PSC, and Pt/C catalyst in a CO2-saturated 1 M KOH solution. (b) Price activity (PA) of each catalyst and price activity at the overpotentials of 100 mV, 200 mV, and 300 mV in a CO2-saturated 1 M KOH solution. (c) Current density at an overpotential of 150 mV and 300 mV for PSC, Pt/C, Non CF-Ru@PSC, and CF-Ru@PSC.
“This work provides a unique and facile synthetic process for the efficient electrocatalyst for an aqueous Zn–CO2 system,” says Professor Kim. “Besides, it can be produced at a cost of one-tenth of the existing Pt‐based electrocatalysts, since it uses Ru and CO2 as raw materials.” He adds, “This facile synthesis progress can also be extended to other active metals for practical applications, and thus further accelerate the commercialization of the previously reported aqueous Zn or Al–CO2 system.”
This study has been also participated by Research Professor Javeed Mahmood (Shool of Energy and Chemical Eingeering, UNIST) and Professor Jeong Woo Han from POSTECH. The findings of this research have been published in the online issue of the Journal Materials Chemistry A on May 29, 2020. It has been also selected as front cover page of the journal and is going to be published soon.
Journal Reference
Jeongwon Kim, Yejin Yang, Arim Seong, et al., “Identifying the electrocatalytic active sites of a Ru-based catalyst with high Faraday efficiency in CO2-saturated media for an aqueous Zn–CO2 system,” J. Mater. Chem. A, (2020), Advance Article.