Researchers at UNIST have developed an innovative device designed for precise diagnosis of sleep apnea, utilizing a smartphone application in the convenience of one’s own home.
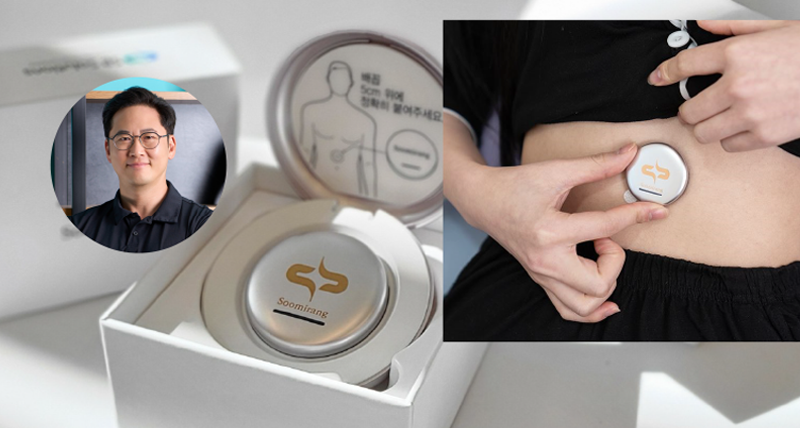
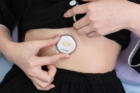
Led by Professor Franklin Bien of the UNIST Department of Electrical Engineering, the team has developed a groundbreaking abdominal-attachable system for diagnosing sleep apnea, employing sensors based on electromagnetic waves embedded within the Soomirang smartphone application. This cutting-edge system can detect changes in a variety of biomarkers through a smart biosensor, yielding a sleep apnea diagnosis with an impressive accuracy rate surpassing 91% when compared to conventional testing methods performed at recognized sleep facilities.
By the mere attachment of the sensor to the abdomen and subsequent analysis of sleep data via a smartphone app, individuals can now independently diagnose sleep apnea, thanks to this innovative technology that seamlessly operates from sensor attachment to real-time data analysis powered by artificial intelligence (AI).
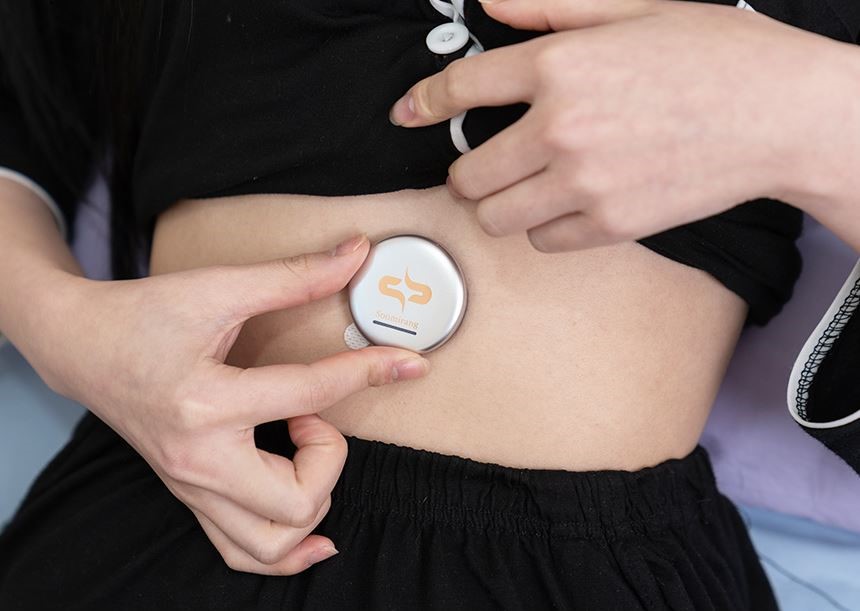
The image above shows Soomirang attached to the abdomen, a device designed to collect biometric data and analyze sleep patterns. l Image Credit: Professor Franklin Bien (Department of Electrical Engineering, UNIST)
Treating sleep apnea has posed challenges due to the inconvenience and high costs associated with conventional testing methods. Patients previously had to endure a night at a designated sleep center or hospital with multiple sensors attached to monitor their condition, resulting in both discomfort and financial burden. Moreover, health insurance policies in Korea further complicated matters by restricting individuals to only one sleep test per year.
However, this new diagnostic system offers a more accurate and convenient approach for individuals to monitor their sleep health, showcasing the potential for advancements in the field of sleep medicine through its detection of sleep apnea using smart biosensor technology. With its compact design and user-friendly interface, the system has the potential to improve the lives of individuals struggling with sleep disorders by providing prompt and precise diagnosis and treatment options.
Following the successful development of this cutting-edge sleep health monitoring technology, the research team obtained approval from the Ministry of Food and Drug Safety (MFDS) in a record 7 months, a process that typically takes around 18 months for a diagnostic assistive medical device. This expedited approval underscores the technological excellence and potential of the system recognized by regulatory authorities.

Image Credit: Professor Franklin Bien (Department of Electrical Engineering, UNIST)
Professor Bien, emphasizing the significance of commercialization in scientific research, expressed, “Our research aims to enhance individuals’ health and well-being through innovative biosensor technology. The approval from the Ministry of Food and Drug Safety is a major milestone in establishing the credibility of our technology.” He further highlighted the importance of applying science and technology in practical contexts to realize their true value.
SB Solutions Inc., a tech startup associated with UNIST and founded by Professor Bien, is actively engaged in commercializing various medical devices through collaborative development. The advancement of abdominal-attachable sleep apnea diagnostic technology is expected to make a significant contribution to enhancing public health and improving sleep quality.