Eukaryotic cells have various organelles, including the nucleus. During cell division (mitosis), researchers for the first time have discovered how proteins regulate the inheritance of lysosomes, which are organelles responsible for digestion.
A research team, led by Professor Changwook Lee from UNIST’s Department of Life Sciences conducted joint research with Professor Youngsoo Jun’s team from Gwangju Institute of Science and Technology (GIST). Using a yeast model, they found that Vac8, a lysosomal protein involved in autophagy, forms a complex with Vac17 to inhibit autophagy and regulate lysosomal transport to divided cells. This discovery is expected to be an important basis for future treatment research on diseases caused by cell division abnormalities such as cancer and chronic conditions.
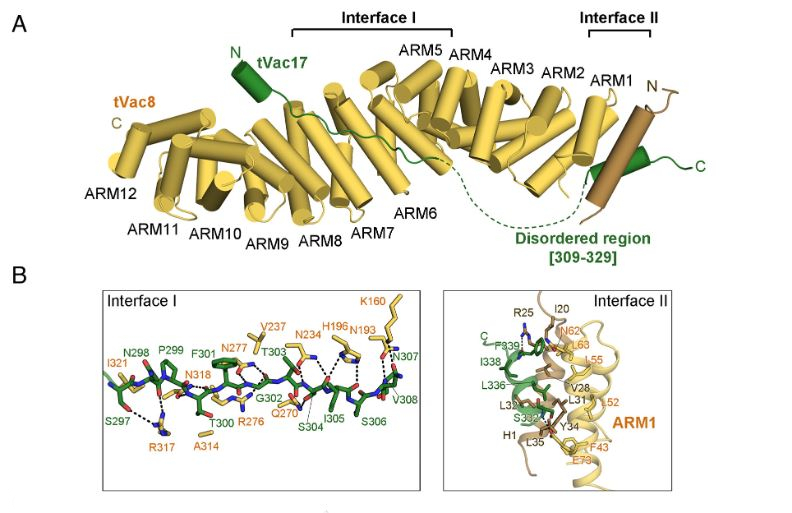
Figure 1. Crystal structure of the tVac8–tVac17 complex. (A) Crystal structure of yeast tVac8 (yellow) in complex with tVac17 (green). (B) Close-up view of the interfaces of the tVac8–tVac17 complex. In Interface I, the tVac17 peptide is drawn as a ball-and-stick representation in green, with oxygen and nitrogen atoms colored red and blue, respectively. Vac8 residues involved in the interaction with Vac17 are shown in yellow. In Interface II, the Hc helix of Vac17 (green) mainly makes hydrophobic contact with the H1 helix (brown) and ARM1 helices (yellow). Black dashed lines indicate intermolecular hydrogen bonds.
Cell division is a crucial process for maintaining life, including in humans. It is finely regulated by various intracellular mechanisms. Intracellular autophagy regulation, in particular, plays a vital role in ensuring complete and safe cell division. This is because if cell organelles and components that divide due to autophagy are decomposed, normal daughter cells cannot form.
Vac8 is a lysosomal protein that selectively regulates autophagy by forming complexes with multiple binding partners. It also works with Vac17 and Myo2 proteins to transport lysosomes during cell division. The Vac8-Vac17-Myo2 complex forms a triple structure that transports lysosomes from the mother cell to the daughter cell through an actin protein acting as an intracellular road.
While previous studies have shown the importance of Vac8-Vac17 complex formation for lysosome transport, molecular-level insights into how Vac8 inhibits intracellular autophagy while performing its lysosome transport functions remain unclear.
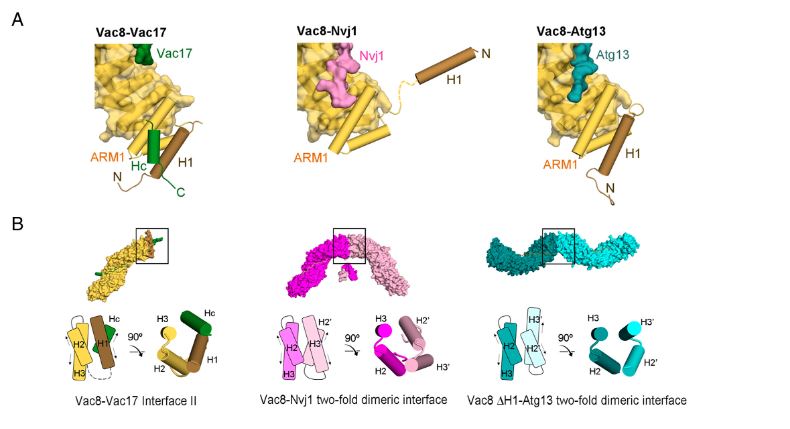
Figure 2. Vac17 is required for the interaction between the H1 helix and ARM1 of Vac8. (A) Structural comparison highlighting the H1 helix and ARM1 of Vac8 in the Vac8–Vac17 (Left), Vac8–Nvj1 (Middle), and Vac8–Atg13 (Right) complexes. The color scheme is the same as in Fig. 4A. (B) Surface representations show the crystal structures of the Vac8–Vac17, Vac8–Nvj1 (PDB ID: 5XJG), and Vac8 (ΔH1)–Atg13 (PDB ID: 6KBN) complexes in the crystallographic asymmetric unit.
The research team used X-ray crystallography to determine high-resolution structures of the Vac8-Vac17 complex for the first time. The said complex has two binding interfaces, with specific amino acids involved in their formation. Mutated amino acids prevented lysosome movement during yeast cell division where both proteins were present – proving that Vac8 protein regulates lysosomal transport.
The study demonstrated how a quaternary structure formed when Vac8 binds to specific partner proteins distinguishes between autophagy and lysosomal transport. The structural differences are regulated through the H1 helix of ARM domain and N-terminal contained in Vac8. During the formation of Vac8-Vac17 complex, Vac17 binds to H1 helix of Vac8 and prevents its separation from ARM domain, suppressing autophagy-causing complex formation. Therefore, during cell division, lysosomal transport occurs while autophagy is suppressed.
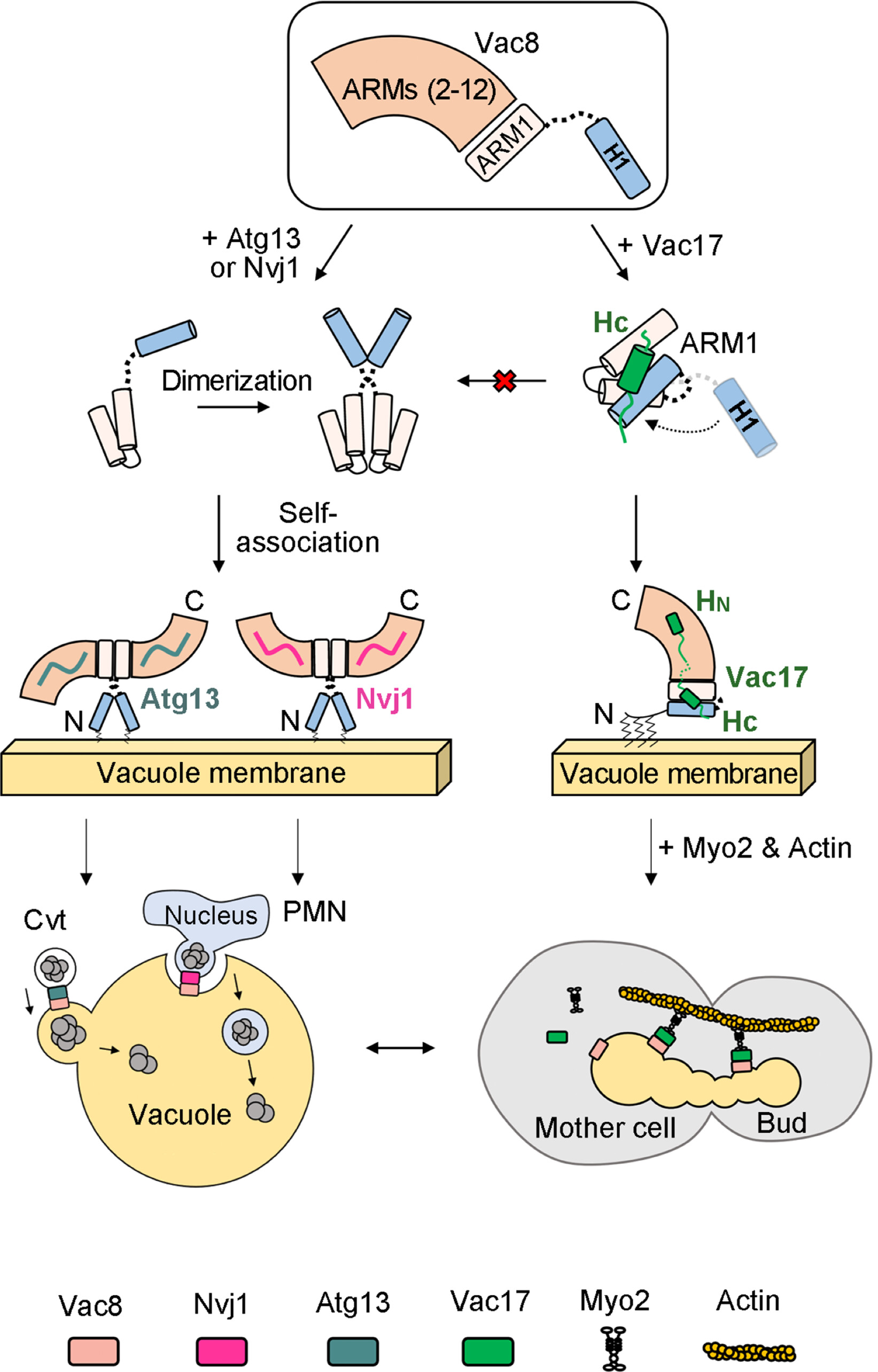
Figure 3. A working model of how Vac8 mediates vacuole inheritance in comparison with the Cvt and PMN pathways.
“This study is an important example of how a single protein can control lysosomal transport during cell division,” noted Professor Lee. “It will be a significant contribution towards research on cancer and various chronic diseases related to cell division.”
The findings of this research have been published in the April 2023 issue of Proceedings of the National Academy of Sciences (PNAS). This study has been supported by the National Research Foundation of Korea (NRF) and the Ministry of Science and ICT (MSIT).
Journal Reference
Hyejin Kim, Jihyeon Park, Hyunwoo Kim et al., “Structures of Vac8-containing Protein Complexes Reveal the Underlying Mechanism by Which Vac8 Regulates Multiple Cellular Processes,” PNAS (2023).













