A research team, unveiled a noble technology, capable of extracting hydrogen (H₂) stored in ammonia (NH₃) by adding silicon (Si), simultaneously producing high-purity H2 and silicon nitride (Si₃N₄). This innovative process not only reduces hydrogen production costs, but also enables the recycling of Si from waste solar panels, attracting significant attention as a sustainable solution.
Led by Professor Jong-Beom Baek in the School of Energy and Chemical Engineering at UNIST, the research team announced the development of a ball milling process that can produce 100% pure H₂ directly directly from NH₃ at low temperatures.
NH3 is regarded as a promising clean fuel carrier because of its high H₂ content (17.6 wt%) and well-established infrastructure for storage and transport. However, conventional methods to release H₂ from ammonia require high temperatures (400–600°C) and additional purification steps, leading to increased energy consumption and costs.
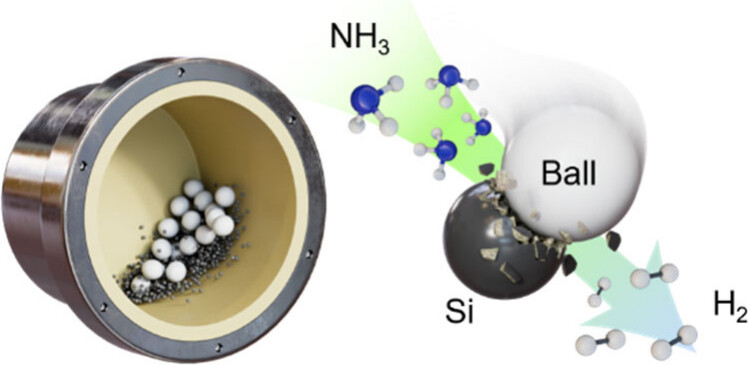
Figure 1. Schematic representation of the mechanochemical NH3–Si (MAS) reaction.
The newly developed process operates at a remarkably low temperature of around 50°C, significantly reducing energy requirements. It involves placing ammonia gas and finely powdered silicon into a sealed container known as a ball mill—containing small ceramic or steel beads—and vigorously shaking it. The mechanical impact and friction activate the silicon, rapidly decomposing ammonia to release H₂. During this process, nitrogen (N₂) is also produced but reacts with silicon to form Si₃N₄, which remains in the system rather than escaping as a gas.
Experimental results demonstrated complete ammonia decomposition, generating H₂ at a rate of 102.5 mmol per hour, with purity confirmed at 100%—free from nitrogen or other impurities. Notably, when using silicon recovered from end-of-life solar panels, the process achieved the same high conversion efficiency and H₂ purity.
Si₃N₄, a high-value material produced as a byproduct, has promising applications in secondary batteries. Lithium-ion batteries incorporating the synthesized silicon nitride achieved a capacity of 391.5 mAh/g, maintaining over 80% of their initial capacity after 1,000 charge-discharge cycles with a Coulombic efficiency of 99.9%.
Economic analyses indicate that, when accounting for revenue from selling Si₃N₄ derived from waste solar panels, the cost of H₂ production could be negative—around -7.14 USD per kilogram—making the process potentially profitable and environmentally beneficial.

Figure 2. Concept diagram of a process for separating H2 from NH3.
Professor Baek stated, “This development offers a solution to the longstanding challenge of H₂ separation and purification in ammonia-based H₂ economy.” He further added, “Using Si recovered from waste solar panels, the process performs comparably to using commercial silicon powder, demonstrating its viability as a sustainable recycling technology. It could play a significant role in managing the over 80 million tons of photovoltaic waste projected by 2050.”
This research was published in the September 2025 edition of the Journal of the American Chemical Society (JACS). The project was supported by funding from the Korea Research Foundation, the Ministry of Science and ICT, and the Korea Institute for Industrial Technology Evaluation and Planning.
Journal Reference
Seung-Hyeon Kim, Runnan Guan, Jiwon Gu, et al., “Separation-Free High-Purity Hydrogen Production via the Mechanochemical Ammonia–Silicon Reaction under Mild Conditions,” JACS, (2025).