A joint research team, affiliated with UNIST has unveiled a novel method of converting nitric oxide (NO), which is the main cause of fine dust, into pure ammonia (NH3) without emitting toxic gases, such as carbon dioxide (CO2). This breakthrough has been jointly led by Professor Youngkook Kwon and Professor Hankwon Lim in the School of Energy and Chemical Engineering at UNIST, in collaboration with Professor Hyungjun Kim from KAIST.
In this study, the research team represented the electrochemical reduction of nitric oxide (NO) on a nanostructured Ag electrode in combination with a rationally designed electrolyte containing the EDTA–Fe2+ metal complex (EFeMC), which results in an ∼100% efficiency for NH3, without any degradation in catalytic activity or product selectivity up to 120 hr.

Figure 1. Their findings have been featured as the cover page of the November 2020 issue of ACS Energy Letters.
The existing electrochemical conversion techniques are associated with the relatively slow rates of chemical reaction, as nitric oxide (NO) does not dissolve very well in the electrolyte. Besides, it has the disadvantage of poor usability due to the formation of undesired side reactions (i.e., N-N coupling), as well as additional by-products (i.e., Nigrogen or N2).
The newly-developed technique has also overcome the disadvantages of the conventional Haber–Bosch process, a process that is energy- and capital-intensive and raises serious environmental concerns due to high CO2 emission as a byproduct. In addition, it is expected to reduce the economic and construction burden of complex facilities for high-temperature and high-pressure.
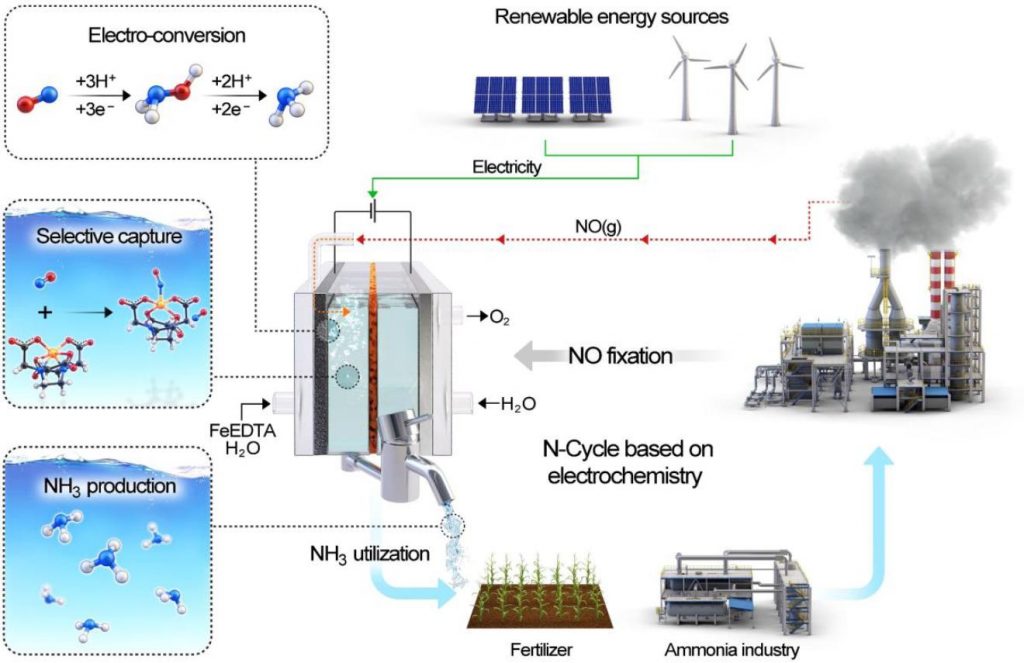
Figure 2. Schematic diagram showing NO capture by EFeMC present in the electrolyte and its electrochemical reduction to ammonia.
According to the research team, the new electrochemical system showed an ∼100% efficiency for NH3 without any degradation in catalytic activity or product selectivity up to 120 h. “Not only that economic analysis using itemized cost estimation predicted that the synthesis of ammonia from NO reduction in an EFeMC-designed electrolyte can be market competitive at an electricity price of $0.03 kWh–1 with a current density of >125 mA/cm2,” noted the research team. “Therefore, this approach will open an entirely new avenue of renewable electricity-driven ammonia synthesis.”
The findings of this research have been featured as the cover page of the ACS Energy Letters on November 13, 2020. This work has been supported by the National Research Foundation (NRF) of Korea, UNIST, and Ulsan Metropolitan City Hall.
Journal Reference
DongYeon Kim, Dongyup Shin, Juheon Heo, et al., “Unveiling Electrode–Electrolyte Design-Based NO Reduction for NH3 Synthesis,” ACS Energy Letters, (2020).