RecensMedical Inc., founded by Professor Gun-Ho Kim of the Department of Mechanical Engineering at UNIST, is pleased to announce that the U.S. Food and Drug Administration (FDA) has granted De Novo approval to OcuCool®, a revolutionary rapid cooling anesthesia designed for painless ophthalmology injection therapy. This innovative device is reportedly the first medical device from Korea to receive FDA approval, marking a significant advancement in patient comfort during ocular treatments.
The FDA’s De Novo process provides a streamlined approval pathway for medical devices that offer new technologies lacking prior market equivalents. Through this rigorous evaluation, OcuCool® has received notable acclaim for its potential as a promising alternative to traditional chemical anesthesia in invasive eye procedures.
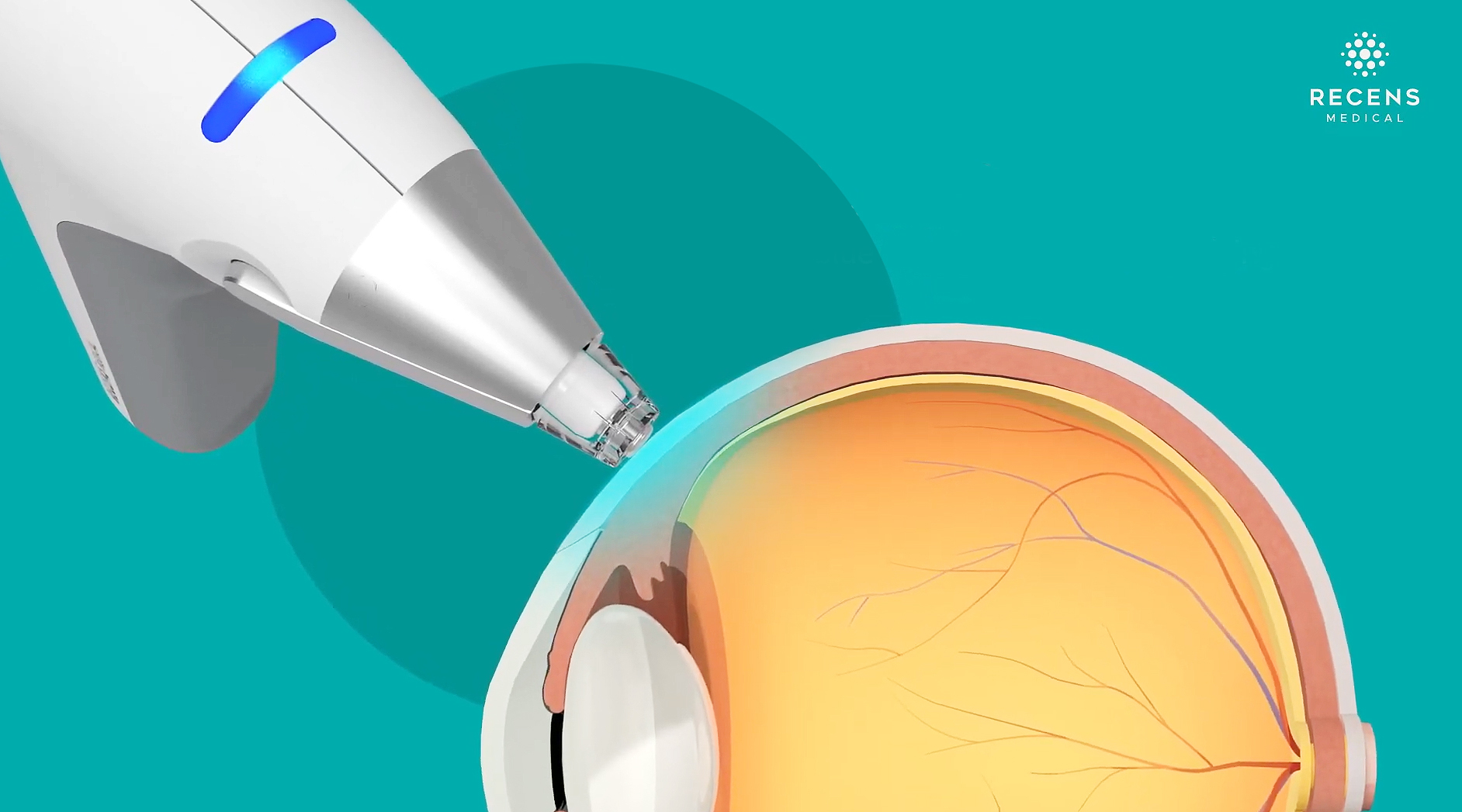
RecensMedical’s OcuCool, the first Korean medical device to receive FDA De Novo approval, uses rapid precision cooling technology to provide anesthesia within 10 seconds for invasive eye treatments. Image Credit: RecensMedical Inc.
OcuCool® utilizes precise cooling technology to safely block nerve signals, achieving anesthesia within just 10 seconds of contact. In contrast, existing chemical anesthetics typically require approximately 5 to 10 minutes to take effect. As a result, the overall duration of the procedure has been significantly reduced from an average of 10 to 15 minutes down to only 1 to 2 minutes. This substantial decrease in treatment time minimizes exposure to chemical agents, thereby significantly reducing side effects such as redness, stinging, and dryness.
Furthermore, OcuCool® is considered safer and more effective than conventional intravitreal injection procedures (IVT) for treating patients with severe eye diseases, including macular degeneration and diabetic retinopathy.
To confirm its safety and efficacy, OcuCool® underwent clinical trials at 10 ophthalmology hospitals in the U.S. from 2018 to 2022, successfully completing phases 1, 2, and 3. Remarkably, 80 percent of trial participants expressed a preference for OcuCool’s cooling anesthesia over traditional anesthetics, as they experienced reduced procedure times and fewer side effects.
“Pharmacological anesthesia requires time to take effect as the molecules must chemically diffuse through nerve ion channels, whereas cooling anesthesia is activated by physical cooling, resulting in a much faster onset,” explained Dr. Charles C. Wykoff, a surgical retina specialist and director of research for Retina Consultants of Houston, who participated in the early clinical trials. He further noted, “We observed a positive response from patients, driven by reduced waiting times and faster recovery following injection procedures compared to conventional methods.”
With the aging population and the increasing prevalence of diabetes, the market for IVT is expected to grow significantly. According to market research firm QY Research, the global IVT market was valued at approximately $13.7 billion in 2020 and is projected to reach around $22 billion by 2027, with an average annual growth rate of about 7 percent.

Professor Gun-Ho Kim, Department of Mechanical Engineering at UNIST, founder of RecensMedical Inc. l Image Credit: RecensMedical Inc.
“We are extremely proud that OcuCool’s FDA De Novo approval validates RecensMedical’s leadership in rapid precision cooling technology,” stated Professor Kim, CEO of RecensMedical. He expressed optimism that OcuCool’s innovation will lead to faster, more comfortable treatments for patients with vision-threatening conditions, thereby enhancing the quality of hospital services. He added, “As a company dedicated to pioneering innovative technologies—not just a follower—we aim to showcase the excellence of Korean medical devices and contribute to improved outcomes for both patients and medical staff through our advanced products.”
Founded in 2016, RecensMedical Inc. has also developed TargetCool, a precision cooling device for dermatology, which is currently marketed in 29 countries. The company is preparing to expand into the hair loss and animal/home-use medical device markets, leveraging its unique technology. Given the growth potential of its flagship products, the company plans to pursue a Kosdaq IPO via the special technology evaluation program in the coming year.
This achievement underscores UNIST’s commitment to demonstrating that university research can lead to practical innovations in the industrial sector. Moving forward, UNIST intends to enhance its support for startups and the commercialization of technology.












